The invention provides engineered mutant – G168W-CtGH1, of the β-glucosidase enzyme WT-CtGH1, derived from Clostridium thermocellum, which exhibit enhanced glucose tolerance. The mutant was generated through site-directed mutagenesis at the Gly-166 residue with Trp. The engineered enzyme demonstrates improved resistance to glucose inhibition and maintain enzymatic function at industrial operating conditions. The mutant is expressed in bacterial host and will be useful in the enzymatic hydrolysis lignocellulosic mass, facilitating efficient saccharification for bioethanol production.
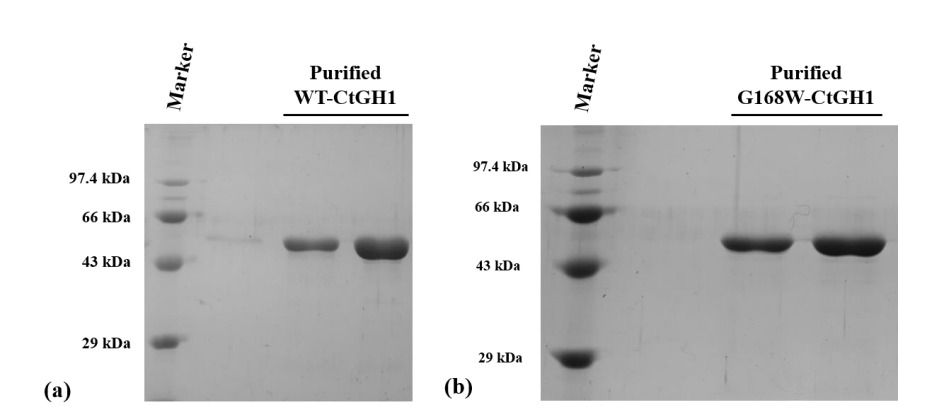
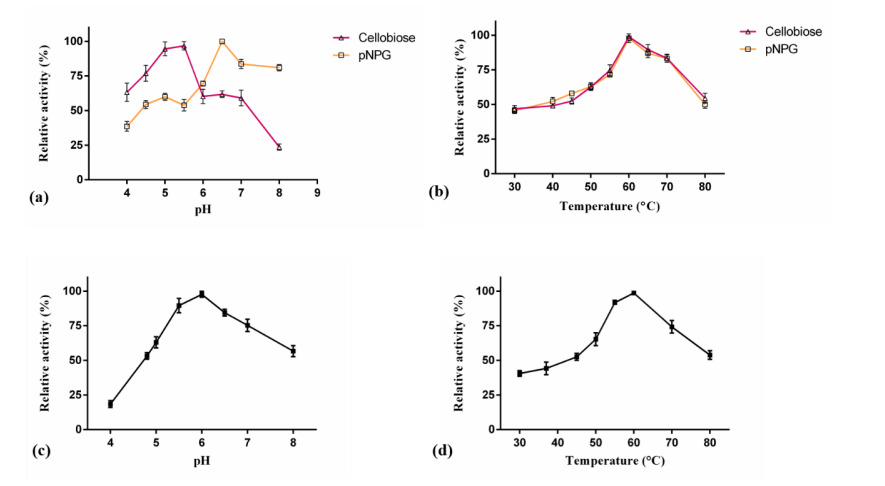
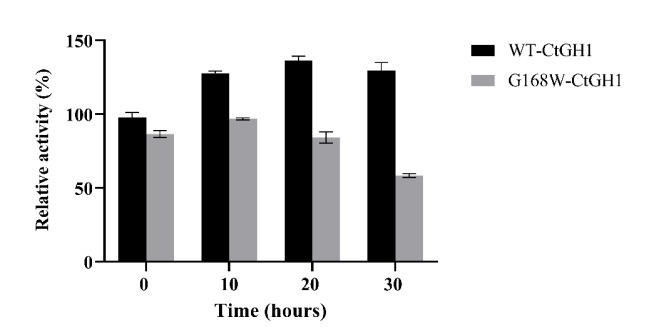
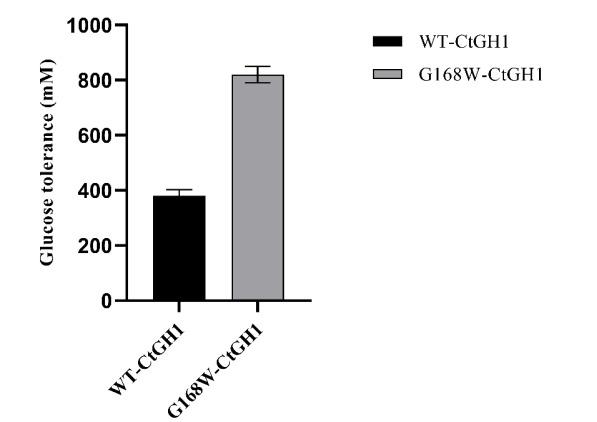
Figure (1) Purification of WT-CtGH1 and G168W-CtGH1 through gel filtration chromatography. SDS-PAGE showing purity of (1a) WT-CtGH1 and (1b) G168W-CtGH1. (2) Biochemical characterization of WT-CtGH1 and G168W-CtGH1, (2a) showing the optimum pH for the activity of WT-CtGH1 at pH 6.0 and 5.5 with pNPG and cellobiose as substrate, respectively, (2b) showing the optimum temperature for the activity of WT-CtGH1 at 60°C with both pNPG and cellobiose as substrate, (2c) showing the optimum pH of 6 for activity of G168W-CtGH1, (2d) showing the optimum temperature for the activity at 60°C for G168W-CtGH1. (3) Thermal stability of WT-CtGH1 and G168W-CtGH1. The bar graph is showing relative activity of WTCtGH1 and G168W-CtGH1 plotted against time of incubation. Assay was performed at 55°C in pH 5.5 buffer with cellobiose as a substrate for WT-CtGH1. The black bars indicate the WT-CtGH1 without glycerol as an additive. Blue line indicates the WT-CtGH1 with 5% glycerol as an additive. The grey bars denote the relative activity of G168W-CtGH1 plotted against time of incubation. Assay was performed at 55°C in pH 5.5 buffer with pNPG as a substrate. (4) Comparison of glucose tolerance between WT-CtGH1 and G168W-CtGH1. The graph shows the relative activity of WT-CtGH1 and G168W-CtGH1 plotted against the increasing concentrations of glucose.
β-glucosidases are inhibited by high concentrations of glucose, their own reaction product, which is accumulated during the enzymatic hydrolysis of lignocellulosic biomass. This product inhibition limits the efficient conversion of cellobiose to glucose, reducing the effectiveness of biomass saccharification and overall glucose yield. Additionally, many existing β-glucosidases lack the thermal stability needed for sustained activity at industrial operating conditions (temperature of 50°C–60°C and pH ranging in 4.5 – 5.5). There is a critical need for β-glucosidase variants that are both glucose-tolerant and stable at industrial operating conditions to improve the efficiency and economic viability of biofuel and biochemical production processes.
- Engineered Enzyme Variants: The β-glucosidase from Clostridium thermocellum (WT-CtGH1) was genetically modified at the G168 (Gly) position to W (Trp) residue, with no loss in the activity of enzyme towards cellobiose.
- Improved Glucose Tolerance: The engineered enzyme – G168W-CtGH1 demonstrates more than two-fold increase in the glucose tolerance (IC50 – 840 mM glucose) compared to the WT-CtGH1 (IC50 – 380 mM glucose).
- Thermal Stability: The mutant β-glucosidases retain stable enzymatic activity within the temperature range of 50°C–60°C and pH 5.5, aligning with conditions used in biomass saccharification processes.
- Microbial Expression System: The mutant enzymes were successfully expressed in E. coli, making the technology suitable for large-scale production of G168W-CtGH1 mutant.
- Enhanced Saccharification Output: Use of the glucose-tolerant variants in saccharification assays would lead to increased glucose release and sustained enzymatic activity, improving overall hydrolysis efficiency.
The glucose-tolerant β-glucosidase mutant – G168W-CtGH1, was developed by introducing specific amino acid substitutions at the Gly-166 position in the WT-CtGH1 from Clostridium thermocellum. The substitution was carried out by site-directed mutagenesis and successfully expressed in Escherichia coli BL21 (DE3) expression host. The recombinant G168W-CtGH1 enzyme was purified and characterized using biochemical assays with para-nitrophenyl-β-D-glucose and cellobiose as substrates. Enzymatic activity of G168W-CtGH1 was tested in the presence of varying glucose concentrations, confirming enhanced tolerance compared to WT-CtGH1. Additionally, stability of engineered G168W-CtGH1 was assessed by incubating the enzyme at 55°C and pH 5.5 and hence was compared with the WT-CtGH1.
The glucose-tolerant G168W-CtGH1 mutant has been successfully generated by site-directed mutagenesis. The mutant was expressed in E. coli BL21 (DE3), and purified. The enzymatic activity, glucose tolerance, and thermal as well as pH stability were validated using biochemical assays. Comparative studies with the WT-CtGH1 confirmed improved glucose tolerance. The lab-scale saccharification of cellulosic biomass using the blend of commercial cellulase cocktail without β-glucosidase and G168W-CtGH1 showed enhanced glucose release. The technology is ready for scale-up.
4
The development of glucose-tolerant and thermally stable β-glucosidase mutants directly supports the efficient conversion of lignocellulosic biomass into fermentable sugars, enabling cost-effective production of second-generation biofuels. By overcoming glucose inhibition – a major bottleneck in enzymatic hydrolysis – this technology promotes greater sugar yield and reduces enzyme loading, lowering operational costs. It contributes to sustainable energy generation, reduces dependency on fossil fuels, and enables better utilization of agricultural waste, thereby aligning with global goals of environmental sustainability and energy security.
- Lignocellulosic biomass hydrolysis, where glucose-tolerant enzymes improve conversion efficiency in the presence of high glucose concentrations.
- Second-generation bioethanol production, using enzyme cocktails with enhanced saccharification performance.
- Industrial enzyme manufacturing, particularly for thermostable and product-tolerant cellulase systems. Biorefinery processes, involving enzymatic breakdown of agricultural residues and organic waste.
- Microbial fermentation industries, where increased sugar availability supports high-yield bio-product synthesis.
Geography of IP
Type of IP
202321028138
548001