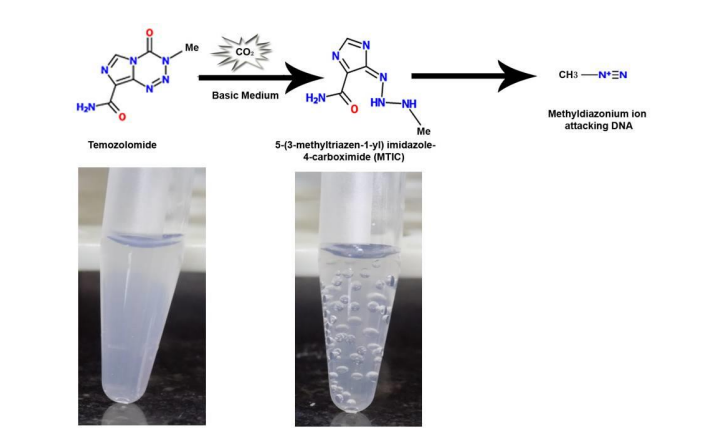
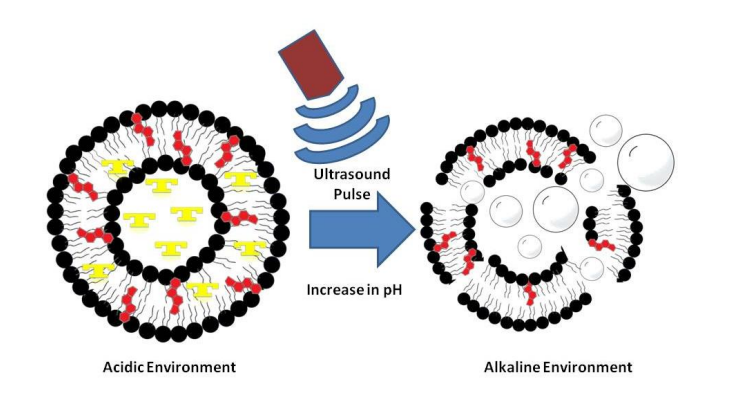
This invention presents prodrug-loaded liposomes that act as in situ theranostic agents for brain tumors like glioblastoma. The liposomes release carbon dioxide upon pH change or ultrasound, enabling both drug delivery and ultrasound imaging without using external gases. Made from soya lecithin, cholesterol, TPGS, and temozolomide, they show high drug loading, biocompatibility, and are suitable for intranasal use. The system allows non-invasive, targeted therapy with real-time monitoring.
Figure (1) Activation of prodrug liposomes containing temozolomide to 5-(3- methyltriazen-1-yl) imidazole-4-carboximide (MTIC) spontaneously initiated by a slightly alkaline pH as in glioblastomas. Photographs of liposome suspensions in microcentrifuge tubes containing temozolomide in acidic medium (left) and in alkaline medium containing carbon dioxide bubbles (right); (2) Mechanism of action through the release of carbon dioxide on activation of temozolomide loaded in liposomes. Liposomes in acidic medium on being subjected to a change in pH and an ultrasound pulse of 1 Watt/ cm2 (spatial average temporal peak) for less than a minute, lead to generation of carbon dioxide that can act as a contrast agent. Liposomes illustrated contain soya lecithin, cholesterol, TPGS and temozolomide in the shell and temozolomide containing buffer in the core.
Current ultrasound-based theranostic drug delivery systems rely on gas-filled microbubbles or perfluorocarbon-loaded nanoparticles, which have limitations such as low drug loading, instability, large size, and poor penetration through biological barriers like the blood-brain barrier (BBB). There is a need for a more efficient, stable, and targeted system for the diagnosis and treatment of brain tumors like glioblastoma multiforme.
- In Situ Gas Generation: The prodrug-loaded liposomes release carbon dioxide upon pH-triggered activation, forming an ultrasound contrast agent without the need for external gas additives.
- Dual Functionality: The system enables simultaneous imaging and targeted drug delivery, functioning as a complete theranostic platform.
- High Encapsulation Efficiency: The liposomes achieve approximately 91.6% encapsulation efficiency with a drug loading capacity of around 30.5%.
- Ultrasound and pH Responsiveness: The liposomes are responsive to both changes in pH and the application of ultrasound, allowing for controlled activation and release.
- Biocompatible and Stable: The formulation avoids the use of perfluorocarbons, exhibits good biocompatibility, and shows enhanced mucoadhesive properties suitable for intranasal administration.
It is prepared using thin-film hydration and probe sonication. TEM and SEM confirmed nanoscale morphology. Liposomes were ~250–300 nm in diameter. Triggered release of drug and CO₂ was validated using ultrasound and pH stimulation.
The technology has been validated by testing the hypothesis through experiments conducted in the laboratory.
4
It enables safer, non-invasive imaging and treatment of brain tumors, improving patient compliance and potentially increasing survival. Reduces systemic chemotherapy toxicity and enhances site-specific delivery.
- Cancer Therapeutics: Enables targeted treatment for glioblastoma with improved effectiveness
- Nanotechnology Drug Delivery: Uses liposomes for precise and controlled drug release
- Biomedical Imaging: Allows real-time ultrasound imaging through in situ CO₂ generation
- Precision Medicine: Supports site-specific, responsive therapy tailored to patients
- Neuroscience & Neuro-Oncology: Enhances drug delivery across the blood-brain barrier
- Diagnostic & Therapeutic Devices: Combines imaging and treatment in a single platform
Geography of IP
Type of IP
201921029504
403693