Many organic and inorganic nanosystems are being actively researched for their efficiency in cancer diagnosis and treatment. Among them, plasmonic nanostructures for photothermal therapy (PTT) gain considerable importance owing to the advent of two on-going PTT based clinical trials making use of gold nanoshells for the treatment of brain and metastatic lung tumors. These plasmonic nanostructures also serve as efficient candidates for imaging, and thereby bringing out their multifunctional capabilities. According to the Food and Drug Administration (FDA) guidelines, any imaging agent (administered into the body) should be capable of getting cleared completely from the body within a reasonable period of time.
Gold-based materials deployed in PTT are generally larger than 20 nm in size. Accumulation of such metallic nanoparticles in body could serve as a potential health risk. Although many of such materials could serve as efficient imaging agents, their larger size and non-degradable nature prevents renal clearance, thus limiting their application in vivo. To achieve renal clearance the size of inorganic nanoparticles will have to be < 5.5 nm. Inorganic, metal containing nanoparticles lesser than 5.5 nm in size are capable of getting filtered through the glomerular basement membrane (GBM), thereby serving as ideal candidates for imaging (with renal route of clearance) but are unsuitable for PTT. Hence, a multifunctional nanosystem capable of achieving good body clearance through both hepato-biliary and renal route in addition to serving as effective agents for PTT is warranted.
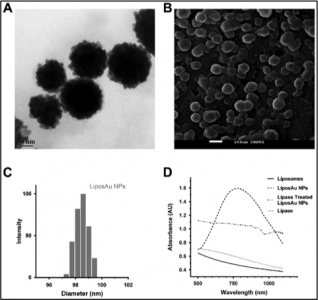
With this objective, thermo-sensitive liposomes were prepared by thin film hydration method followed by gold coating to form Liposome-Au nanoparticles (Lipos-Au NPs). These particles were tuned to have an NIR absorbance of 750 nm that was put to use in optical / CT imaging and photothermal treatment. The pharmacokinetic study of Lipos-Au NPs performed in small animal model indicates in situ degradation in hepatocytes and further getting cleared through the hepato-biliary and renal route. Further, the therapeutic potential of Lipos-Au NPs tested in mouse tumor xenograft model using NIR laser (750 nm) illumination resulting complete ablation of tumor mass, thus prolonging disease-free survival. This project has been extensively covered by several media and publication houses and has been appreciated at several forums. The technology is ready for scale up, preclinical toxicology and clinical trials.
Prof. Rohit Srivastava
