This invention presents a simple and low-cost technique for determining the concentration of sickle-cell haemoglobin (HbS) in blood. The method relies on measuring the length of a blood smear formed at a controlled blade speed. The smear length correlates with the viscosity of the blood, which increases in sickle-cell conditions. The technique enables quantification of HbS concentration using minimal blood volume, and can distinguish between normal, sickle cell trait (SCT), and sickle cell anaemia (SCA) samples.
Diagnosing sickle cell anaemia (SCA) and sickle cell trait (SCT) usually requires advanced tests like HPLC or electrophoresis, which are expensive and need trained staff. These tests are not easily available in rural or low-resource areas. Cheaper methods exist, but they often cannot tell the difference between SCA and SCT. There is a strong need for a quick, low-cost, and easy-to-use method that can accurately measure the amount of sickle haemoglobin (HbS) and help identify both SCT and SCA.
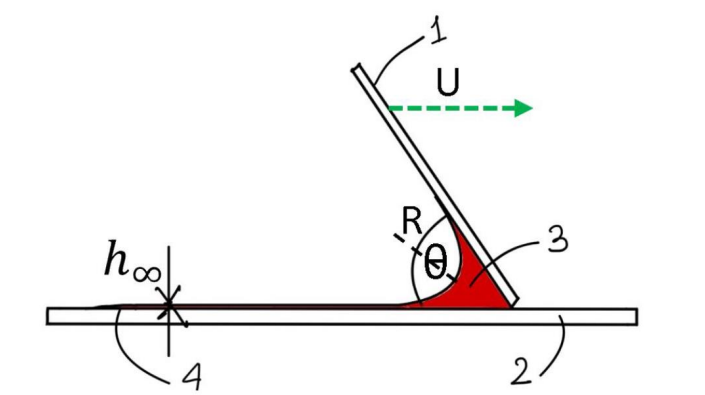
- Smear-Based Quantification: This technique calculates the concentration of sickle-cell haemoglobin (HbS) in blood by analysing the length of a smear formed at a controlled blade speed. The smear length is directly related to the viscosity of the blood, which increases in the presence of higher HbS content.
- Minimal Sample Requirement: The method requires only 5–20 microlitres of blood, making it suitable for use with finger-prick samples. This small volume requirement supports easy adoption in field settings and reduces patient discomfort, especially for repeated tests.
- Differentiation Between SCT and SCA: The technique is capable of distinguishing between sickle-cell trait (SCT) and sickle-cell anaemia (SCA) by comparing the viscosity of blood before and after deoxygenation. A higher percentage increase in viscosity after sickling treatment corresponds to SCA, while a moderate increase indicates SCT.
- Use of Reducing Agent: Sodium metabisulphite is used to chemically reduce the oxygen in the blood sample, mimicking low-oxygen conditions. This induces sickling in red blood cells containing HbS, allowing the test to simulate the physiological behaviour of sickle-cell blood.
- Integrated Visual Tool: The method includes a specially designed SMEAR CARD® with graduated markings, which enables users to visually measure smear length and estimate the corresponding blood viscosity. This provides a simple, instrument-free way to quantify HbS concentration, supporting deployment in low-resource environments.
The prototype consists of a compact, benchtop smearing device that uses a motorized blade to draw blood smears at controlled speeds ranging from 10 to 200 mm/s. The blade operates at a fixed angle (typically between 20° and 45°) to ensure consistent contact with the glass slide. A small volume of blood (5–20 µL), pre-treated with 1% sodium metabisulphite, is placed on the slide before smearing. To measure viscosity, the system uses a specially designed SMEAR CARD®, which has printed graduations to help estimate smear length and corresponding viscosity in centipoise (cP). This allows users to visually interpret results without needing advanced lab equipment. The prototype setup is simple, portable, and uses readily available materials, making it well-suited for point-of-care use in low-resource settings. It supports both fresh and stored blood samples and enables quick testing in under three minutes.
The method has been tested on real patient samples with clear differentiation between healthy, SCT, and SCA conditions. The technology awaits validation from the ICMR, India. The smear technique has been tested using a compact automatic smearing device. A prototype system including the smear card and measurement protocol has been successfully demonstrated.
5
This technique enables accessible screening of sickle cell disease in low-resource environments. It reduces reliance on costly diagnostics while delivering quantitative insights into HbS levels. The system is particularly valuable for rural clinics, public health campaigns, and regions with high SCD prevalence like India and sub-Saharan Africa. It also supports ongoing treatment monitoring and can be extended to drug screening applications.
- Point-of-care diagnostics
- Public health screening programs
- Haematology and pathology lab
- Rural and primary healthcare
- Pharmaceutical R&D (sickle-cell therapy trials)
Geography of IP
Type of IP
202421027804
564546