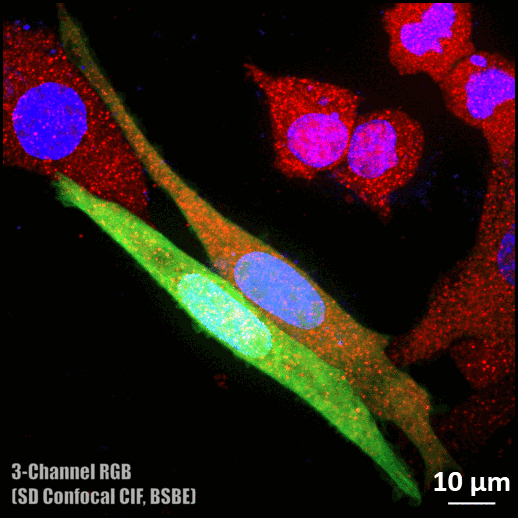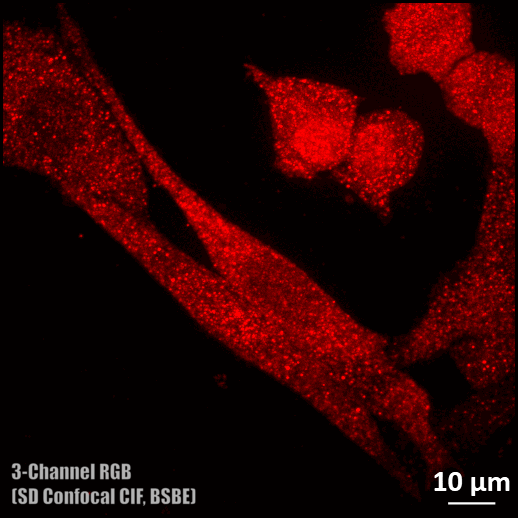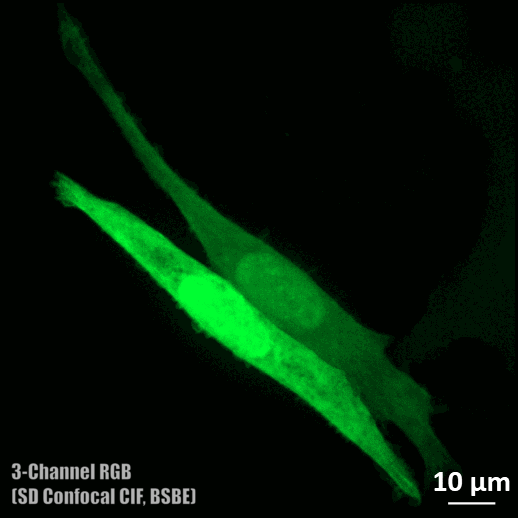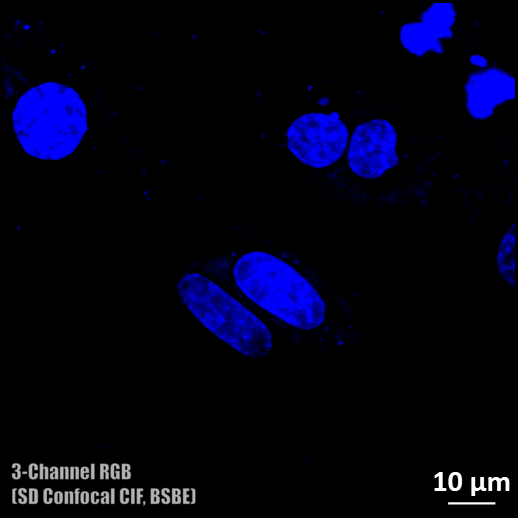
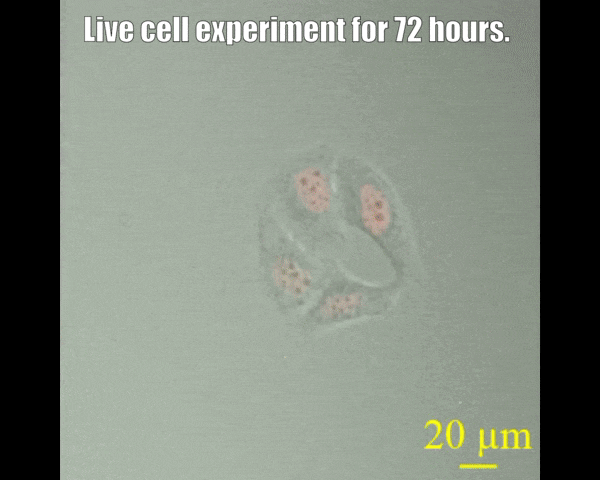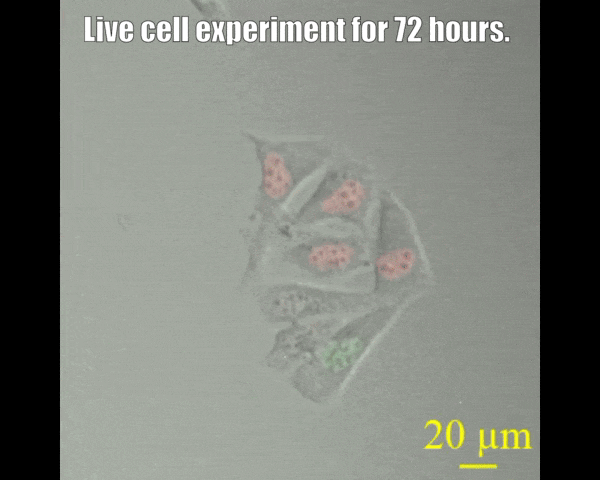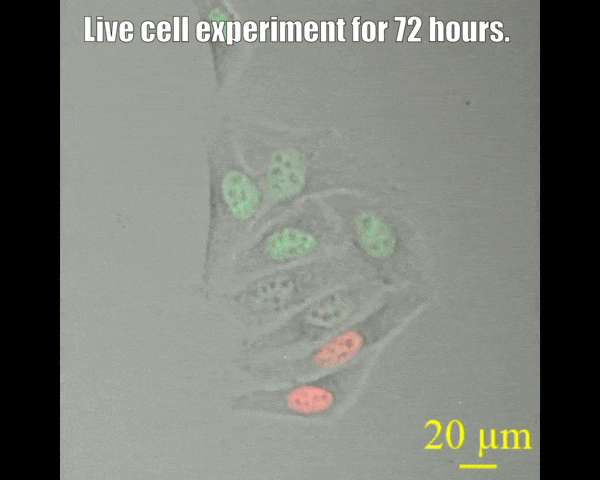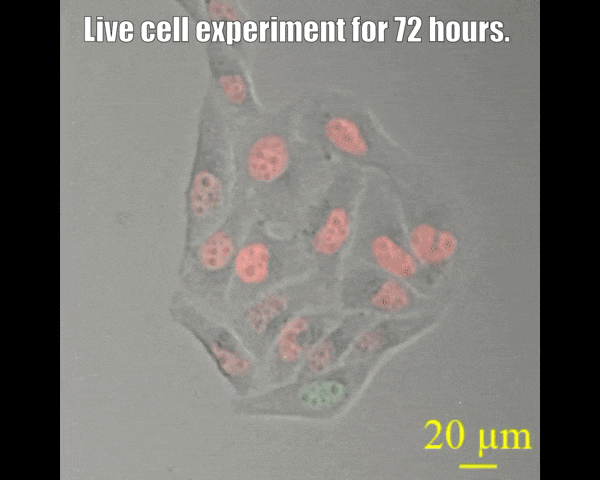
Spinning disc confocal microscope facility
A spinning disk confocal microscope, when coupled with a sensitive electron-multiplying charge-coupled device (EMCCD) camera, can exceed the sensitivity of a laser scanning confocal microscope system. The fast acquisition time of a spinning disk confocal microscope also allows the collection of three- or four-dimensional data that may not be possible on other systems. For example, it takes seconds rather than minutes to acquire data of a 15-μm Z-stack with individual slices separated by 0.25 μm (68 μm by 68 μm field of view). The acquisition time is dictated by the intensity of the fluorescence signal, which depends on protein concentration laser power, and the camera's sensitivity. Since the immobilization of live animals may not be complete, fast acquisition time also minimizes the probability of sample movement during image acquisition. This in turn allows high-quality reconstruction of all signals in a three-dimensional space. The spinning-disk confocal microscope facility is suitable for capturing fast-changing phenomena, such as imaging swimming bacteria, lipid droplets, etc. Most powerful tools for live cell imaging.
- SD Confocal Mode: Three channel/color imaging along with brightfield/DIC imaging Z-stacks, Multipoint, and Time series (with or without Z-stack).
- Fluorescence/Widefield microscope mode: Six channel/color imaging along with brightfield/DIC imaging Z-stacks, Multipoint, and Time series (with or without Z-stack).
- Microscope and spinning disc: Zeiss observer Z1 inverted motorized and computer-controlled fluorescence microscope fitted with high-speed microlens-enhanced nipkow spinning disc.
- Objectives: 10X/0.45 NA (air), 20 X/0.8 NA (air), 40 X/1.2 NA (air), 40 X/1.3 NA (oil), 63 X/1.4 NA (oil) & 100 X 1.4 NA (oil). DIC imaging is possible with all the objectives.
- Lasers: 405nm (50mW), 488nm (50mW), and 561nm (20mW) solid-state lasers as excitation sources.
- HXP 120W metal-halide illuminator for wide-field fluorescence.
- Fluorescence filter sets (wide-field; non-confocal)
- Image acquisition at 50 fps at full resolution.
- Three colour (DAPI, Alexa fluor 488, and Rhodamine) confocal imaging is possible.
- High-speed imaging and versatility.
- Z- stacks and 3D image reconstruction.
- ime series (with or without Z-stack).
- Tile scanning to image different parts of the sample automatically over long durations)
- Multi-point with or without time-lapse imaging
- Co-localization analysis, 3D volume rendering and 3D measurement
- Single-color and three-color imaging and brightfield/DIC imaging.
- Fast dynamic processes.
- Multi-position imaging (up to 100 hours).
Central Instrumentation Room, Ground floor Biosciences & Bioengineering Department. IIT Bombay, Powai, Mumbai - 400 076
Contact No: 022-2159 6746/6719
- Mr. Pradip Shinde
- Mr. Santosh Panigrahi