- TECHNICAL SPECIFICATIONS
- SPECIAL FEATURES
- WORKING PRINCIPLE
- CENTRAL FACILITY WORKSHOP PRESENTATION
- FAQ
- PUBLICATION USING DATA FROM FACILITY
- INSTRUCTION FOR SAMPLE PREPARATION
- INSTRUCTIONS FOR USERS
- INSTRUCTIONS FOR REGESTRATION
Microscope and spinning disc: Zeiss Observer Z1 inverted motorized and computer-controlled fluorescence microscope fitted with high-speed microlens-enhanced Nipkow spinning disc (Yokogawa CSU-X1 automated model). Peltier-cooled monochrome EMCCD camera with 56 fps frame rate at full resolution (512x512) for imaging in confocal mode. 1.4 MP monochrome camera with one-stage Peltier cooling mechanism for imaging in non-confocal (widefield) mode. Temperature (from 3oC to 45oC) and CO2-controlled incubation stage. Stage insert available for glass slides and 35mm Petri dishes. Proprietary Definite Focus from Zeiss with NIR LED Hardware Focus to eliminate drift during long-term imaging. Zeiss ZEN Blue image acquisition software.
Objectives: 10X/0.45 NA (air) WD = 2100um, 20X/0.8 NA (air) WD = 550um, 40X/1.2 NA (air) WD = 250um, 40X/1.3 NA (oil) WD = 200um, 63X/1.4 NA (oil) WD = 190um, 100X/1.4 NA (oil) WD = 170um. DIC and brightfield imaging is possible with all the objectives.
Lasers: 405nm, 488nm, and 561nm solid state lasers as excitation sources.
Filters: for widefield (non-confocal) imaging: DAPI, Alexa fluor 488, Rhodamine, and Cy5 are currently mounted (CFP and YFP filters are in stock).
Cameras: Andor EMCCD Camera is used for SD confocal imaging, while the Zeiss AxioCam CCD MRm Camera is used for the widefield microscope.
- The On-stage incubation system and weather-controlled cage allow for up to 100 hours of live cell imaging with temperature and CO2 control.
- Image acquisition at 50fps at full resolution.
- Suitable for observing fast dynamic cell processes
- The system is also equipped with a Definite Focus nosepiece (fast auto-focus to compensate for focus drift during sample imaging) a hardware LED-based drift compensator, for long-time live cell imaging.
There is a trade-off between image acquisition at high-resolution and at high speed with conventional scanning probe confocal microscopy. This is because each point in the sample plane is scanned in a sequential manner to obtain a 2D image. In a spinning-disk confocal microscope, multiple points within the sample are excited simultaneously. The image is recorded using an array detector like a high-speed sensitive EMCCD camera instead of a point detector (e.g. PMT). This system is suitable for capturing fast-changing phenomena, such as imaging swimming bacteria, etc.
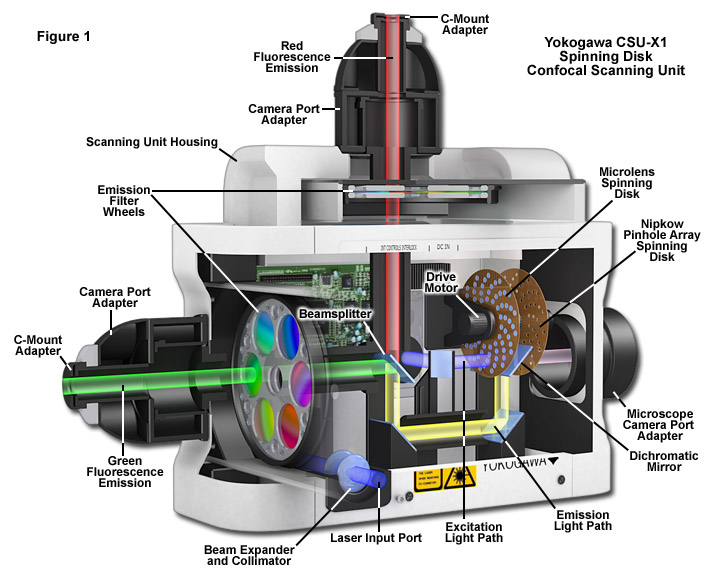
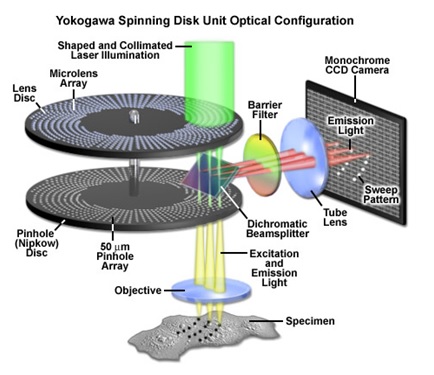
The Yokogawa CSU-X1 spinning disc has a combination of upper and lower disks rotated by a motor. The laser light is first defocused to expand to a larger spot size. This larger laser beam spot is then translated into ~20000 small focused laser beam spots by the upper microlens array disk. These laser beam spots then pass through a dichroic mirror and are perfectly aligned to pass through corresponding pinholes on the lower pinhole array disk. The laser spots are then focused by the objective lens onto the sample. Fluorescent light from the specimen returns along the same path through the objective lens and pinholes, is reflected by a dichroic mirror, and is focused at a camera or eyepiece. Thus, the light beams can illuminate the entire observation area of the specimen and forms a confocal optical slice at the camera or eyepiece.
This FAQ deals with the operational aspects of the facility. If you would like to suggest a question, do feel free to drop an email to sdconfocal@iitb.ac.in
1. I need to do simple slide imaging. Which confocal microscope should I use?
You could use either as long as you image up to three fluorophores (red, green, and blue). If you want to image Far-red (Cy5), you have to use the scanning probe confocal microscope. There is no laser for Far-red (Cy5) excitation in the spinning disc system. If you want to image more than three fluorophores, you need to use the scanning probe confocal microscope.
2.What consumable items should I bring with me? What items will be provided at the facility?
The facility will only provide the immersion oil for the objectives and the lens cleaning tissues. Everything else that you may need during imaging (e.g. gloves, pipettes, tips, regular tissue rolls, aluminium foil, etc.) you will have to bring yourself. If you are in doubt, please speak to the operators or one of the conveners in advance.
3. I need to use the confocal microscope. Do I need to train as a TA?
If your usage is infrequent (less than twice per month), one of the operators or the existing TAs can do the imaging for you. If your research project heavily depends on the use of the confocal facility, it would be better if you trained as a TA. Do remember, training as a TA comes with certain duties, such as, imaging other people’s samples.
4. What does being a TA involve?
The job of a TA is to help us run the facility smoothly and image other people’s samples. You will have a do a minimum of 6 hours of TA duty per week just like the TAs allotted to other central facility equipment. This is non-‐negotiable. If you are a first year PhD student with loads of coursework, we suggest that you come back after a year. The upside is that you will get really proficient in using a state-of-the-art confocal microscope. You will also be able to book slots during ‘off’ hours (between 6pm and 9am) to run samples for yourself or your research group. On the whole it should be a very useful learning experience for you.
5. I think I need to train as a TA. What should I do?
The first thing you should do is to check with your advisor on whether both of you agree with the time commitment. If you are a non-BSBE student, you need to contact the TA coordinator of your department to see if you could be assigned as a TA in the central facility. If the answer to both questions is ‘yes’, send an email to the convener of the microscope where you want to train. We will take over from there.
6. I booked a slot, but my sample is not ready. What should I do?
This can happen once in a while, so don’t worry. Send an email to confocal@bio.iitb.ac.in and call the operator on his mobile phone as soon as you realize that you cannot make it to your slot. This is a matter of courtesy to ensure that other people can use your slot. If this happens too many times, clearly you are not planning your experiments very well and we will take a strict view of it.
7. I need to do live cell imaging. Which microscope should I go for?
If you are imaging swimming bacteria, sperm cells, etc. you should definitely choose the spinning disc system. If your live cell imaging involves four or more fluorophores (unlikely though!), you have to choose the scanning probe confocal system. If your sample is tagged with a single fluorophore (green or red) and you want to image for an hour or so, you can choose either. If you want to do longer experiments (e.g. exploring the motility of a mammalian cell), we will assign you the spinning disc system unless there is a compelling reason to use the other microscope. Such experiments should be scheduled at night (after 6pm). If they need to run longer than 12 hours, you should plan to do these experiments over the weekend. In case of an initial overnight operation, you (or the TA) will need to check on your sample every couple of hours.
8. I need to book more than one consecutive slot. Can I get it?
Sure, if you can justify why. Having ~20 odd samples to image at one go is not a good enough reason. We need to be fair to every user while assigning slots. We will give you as many slots as you need to image all your samples, but they will be distributed over several days.
9. I don’t have any fluorophore in my sample. Can I still use the confocal system?
That depends. If you are doing live cell imaging (with CO2 and temperature) in brightfield/DIC mode for a long enough time, you certainly can use the system, as there is currently no other microscope in IITB that has this facility. Just remember that it won’t be confocal imaging, i.e. you won’t be blocking the out-of-plane light. In such a case, the Definite Focus feature will be very useful to you to ensure that at least one of the Z-stack images remains in focus throughout.
10. Can I request a particular TA to image my sample?
No. All TAs have done the same training and it should not matter who images your sample. The conveners have framed this policy to ensure that no single lab/TA monopolizes the use of the facility. If you have any apprehensions about any TA, feel free bring it to the notice of the convener immediately.
2023-24
- Batra, P. J., Kumari, A., Liao, V. W. Y., Hibbs, D. E., Groundwater, P. W., and Panda, D. (2023) 3,5-bis(styryl)pyrazole inhibits mitosis and induces cell death independent of BubR1 and p53 levels by depolymerizing microtubules. The Journal of Biochemistry. 174, 143–164
- Giri, P., Batra, P. J., Kumari, A., Hura, N., Adhikary, R., Acharya, A., Guchhait, S. K., and Panda, D. (2023) Development of QTMP: A promising anticancer agent through NP-Privileged Motif-Driven structural modulation. Bioorganic & Medicinal Chemistry. 95, 117489
- Kurian, J., Ashtam, A., Kesavan, A., Chaluvalappil, S. V., Panda, D., and Manheri, M. K. (2023) Hybridization of the Pharmacophoric Features of Discoipyrrole C and Combretastatin A-4 toward New Anticancer Leads. ChemMedChem. 18, e202300081
- Rao, V. K., Ashtam, A., Panda, D., and Guchhait, S. K. (2024) Natural-Product-Inspired Discovery of Trimethoxyphenyl-1,2,4-triazolosulfonamides as Potent Tubulin Polymerization Inhibitors. ChemMedChem. 19, e202300562
- Venkatramani, A., Ashtam, A., and Panda, D. (2024) EB1 Increases the Dynamics of Tau Droplets and Inhibits Tau Aggregation: Implications in Tauopathies. ACS Chem. Neurosci. 10.1021/acschemneuro.3c00815
- Sakunthala, A., Datta, D., Navalkar, A., Gadhe, L., Kadu, P., Patel, K., Mehra, S., Kumar, R., Chatterjee, D., Devi, J., Sengupta, K., Padinhateeri, R., & Maji, S. K. (2022). Direct Demonstration of Seed Size-Dependent α-Synuclein Amyloid Amplification. Journal of Physical Chemistry Letters, 13(28), 6427–6438.
- Raza, M. R., George, J. E., Kumari, S., Mitra, M. K., & Paul, D. (2023). Anomalous diffusion of E. coli under microfluid ic confinement and chemical gradient. Soft Matter, 19(34), 6446–6457.
2022-23
- Venkatramani, A., S. Mukherjee, A. Kumari, and D. Panda, Shikonin impedes phase separation and aggregation of tau and protects SH-SY5Y cells from the toxic effects of tau oligomers. Int J Biol Macromol, 2022. 204: p. 19-33.
- Siddiquie, R.Y., A. Gaddam, A. Agrawal, S.S. Dimov, and S.S. Joshi, Anti-Biofouling Properties of Femtosecond Laser-Induced Submicron Topographies on Elastomeric Surfaces. Langmuir, 2020. 36(19): p. 5349-5358.
- Pradhan, A., S. Mishra, A. Surolia, and D. Panda, C1 Inhibits Liquid-Liquid Phase Separation and Oligomerization of Tau and Protects Neuroblastoma Cells against Toxic Tau Oligomers. ACS Chem Neurosci, 2021. 12(11): p. 1989-2002.
- Pradhan, A., S. Mishra, S.M. Basu, A. Surolia, J. Giri, R. Srivastava, and D. Panda, Targeted nanoformulation of C1 inhibits the growth of KB spheroids and cancer stem cell-enriched MCF-7 mammospheres. Colloids Surf B Biointerfaces, 2021. 202: p. 111702.
- Mukherjee, S. and D. Panda, Contrasting Effects of Ferric and Ferrous Ions on Oligomerization and Droplet Formation of Tau: Implications in Tauopathies and Neurodegeneration. ACS Chem Neurosci, 2021. 12(23): p. 4393-4405.
- Kumari, A., O. Shriwas, S. Sisodiya, M.K. Santra, S.K. Guchhait, R. Dash, and D. Panda, Microtubule-targeting agents impair kinesin-2-dependent nuclear transport of beta-catenin: Evidence of inhibition of Wnt/beta-catenin signaling as an important antitumor mechanism of microtubule-targeting agents. FASEB J, 2021. 35(4): p. e21539.
- Sane, A., S. Sridhar, K. Sanyal, and S.K. Ghosh, Shugoshin ensures maintenance of the spindle assembly checkpoint response and efficient spindle disassembly. Mol Microbiol, 2021. 116(4): p. 1079-1098.
- Saha, R., S. Patkar, D. Maniar, M.M. Pillai, and P. Tayalia, A bilayered skin substitute developed using an eggshell membrane crosslinked gelatin-chitosan cryogel. Biomater Sci, 2021. 9(23): p. 7921-7933.
- Patwardhan, S., P. Mahadik, O. Shetty, and S. Sen, ECM stiffness-tuned exosomes drive breast cancer motility through thrombospondin-1. Biomaterials, 2021. 279: p. 121185.
- Malankar, G.S., A. Sakunthala, A. Navalkar, S.K. Maji, S. Raju, and S.T. Manjare, Organoselenium-based BOPHY as a sensor for detection of hypochlorous acid in mammalian cells. Anal Chim Acta, 2021. 1150: p. 338205.
2021-22
- S. Chavan and H. Bagla, Alpha track detection study on CR-39 from granitic wastes employing tetraethyl ammonium bromide as chemical etchant. Journal of Radioanalytical and Nuclear Chemistry, 2020.
- P. Vashisth, N. Kar, Deepak G. and J. Bellare Three Dimensional Quercetin-Functionalized Patterned Scaffold: Development, Characterization, and In Vitro Assessment for Neural Tissue Engineering ACS Chemical Neuroscience, 2020.
- N. Mundhara, A. Majumder and D. Panda, Hyperthermia induced disruption of mechanical balance leads to G1 arrest and senescence in cells. Biochem J., 2021.
- V. Liao, et al., Tubulin-Binding 3,5-Bis(styryl)pyrazoles as Lead Compounds for the Treatment of Castration-Resistant Prostate Cancer. Molecular Pharmacology, 2020.
- A. Navalkar, S. Pandey, N. Singh, K. Patel, B. Mohanty, S. Jadhav, P. Chaudhari and SK. Maji (2020), Direct evidence of cellular transformation by prion-like p53 amyloid infection. J. Cell Science, 2020.
- S. Ray, N. Singh, R. Kumar, K. Patel, S. Pandey, D. Datta, J. Mahato, R. Panigrahi, A. Navalkar, S. Mehra, L. Gadhe, D. Chatterjee, AS Sawner, S. Maiti, S. Bhatia, J. Gerez, A. Chowdhury, A. Kumar, R. Padinhateeri, R. Riek, G. Krishnamoorthy and SK. Maji, (2020), α‐Synuclein aggregation nucleates through liquid-liquid phase separation. Nature Chemistry , 2020.
- J. S. Rane, A. Kumari and D. Panda, The Acetyl Mimicking Mutation, K274Q in Tau, Enhances the Metal Binding Affinity of Tau and Reduces the Ability of Tau to Protect DNA. ACS Chemical Neuroscience, 2020.
- S. Mehra, S. Ahlawat, H. Kumar, N. Singh, A. Navalkar, K. Patel, P. Kadu, R. Kumar, NN. Jha, JB. Udgaonkar, V. Agarwal and SK. Maji (2020), α-Synuclein aggregation intermediates form fibril polymorphs with distinct prion-like properties. bioRxiv, 2020.
- N. Singh, K. Patel, A. Navalkar, P. Kadu, D. Datta, D. Chatterjee, A. Shaw, S. Jadhav and SK. Maji. Amyloid fibril-based hydrogels for high-throughput tumor spheroid modeling. bioRxiv. 2020.
2020-21
- Chavan, S. and H. Bagla, Alpha track detection study on CR-39 from granitic wastes employing tetraethyl ammonium bromide as chemical etchant. Journal of Radioanalytical and Nuclear Chemistry, 2020.
- Das, A., et al., Nuclear softening is essential for protease-independent migration. Matrix Biology, 2019.
- Gupta, D., et al., Multiscale Porosity in Compressible Cryogenically 3D Printed Gels for Bone Tissue Engineering. ACS Applied Materials & Interfaces, 2019.
- Kumari, A., et al., C12, a combretastatin-A4 analog, exerts anticancer activity by targeting microtubules. Biochemical Pharmacology, 2019.
- Liao, V., et al., Tubulin-Binding 3,5-Bis(styryl)pyrazoles as Lead Compounds for the Treatment of Castration-Resistant Prostate Cancer. Molecular Pharmacology, 2020.
- Pradhan, A., et al., Quercetin Encapsulated Biodegradable Plasmonic Nanoparticles for Photothermal Therapy of Hepatocellular Carcinoma Cells. ACS Applied Bio Materials, 2019.
- Rane, J.S., A. Kumari, and D. Panda An acetylation mimicking mutation, K274Q, in tau imparts neurotoxicity by enhancing tau aggregation and inhibiting tubulin polymerization. The Biochemical journal, 2019.
- Rane, J.S., A. Kumari, and D. Panda, The Acetyl Mimicking Mutation, K274Q in Tau, Enhances the Metal Binding Affinity of Tau and Reduces the Ability of Tau to Protect DNA. ACS Chemical Neuroscience, 2020.
- Sawant, A., et al., Crocin, a carotenoid, suppresses spindle microtubule dynamics and activates the mitotic checkpoint by binding to tubulin. Biochemical Pharmacology, 2019.
- The required techniques depend on the type of samples (biological samples, material samples) as well as on the application.
- Currently, we can image fixed samples sealed between a glass slide and a cover slip. Do not bring samples without sealing them with a cover slip.
- 35 mm diameter Petri dishes. Please use specially available imaging Petri dishes with coverslip bottoms if you wish to use oil immersion objectives.
- Multi-well plate dishes. Please use a specially available imaging multi-well plate with glass bottoms if you wish to use oil immersion objectives.
- Users should know what kind of sample preparation is required for his/her samples.
- Please mention what fluorophores you have used in your sample (excitation/emission spectra) when you make a request.
- Users must be available throughout the imaging.
- Only online registration through the IRCC webpage will be accepted. If you need to cancel your slot, send an email immediately with an explanation.
- Slots will be provided on a first-come first-served basis.
- The slots are from 9 am - 11 am, 11 am - 1 pm, 2 pm - 4 pm, 4 pm - 6 pm. You can request two consecutive slots only once a week. If your experiment needs more time (e.g. A long time live cell imaging, etc.), please drop an email to sdconfocal@iitb.ac.in or confocal002@gmail.com and CC Prof. Swati Patankar patankar@iitb.ac.in so that we can deal with your specific requirement./li>
- The non-office hours slots are of 3 hours and it starts from 6 pm to the next day 9 am. (6 pm - 9 pm, 9 pm - 12 am, 12 am - 3 am, 3 am - 6 am, and 6 am - 9 am)
- USB drives are strictly not allowed for copying data to minimize virus-related issues. The data can be shared in the cloud or you need to bring a new blank CD/DVD to transfer your data. All data must be transferred within 15 days of imaging. Without exception.
- • Register online through the IRCC webpage. Link- https://drona.ircc.iitb.ac.in/ircc/NewFac/CentralFacilities.jsp
- • After the slot booking request is accepted, please contact the operator (Mr. Pradip Shinde 022-2159 6746/6719) to discuss the details of your experiment.
