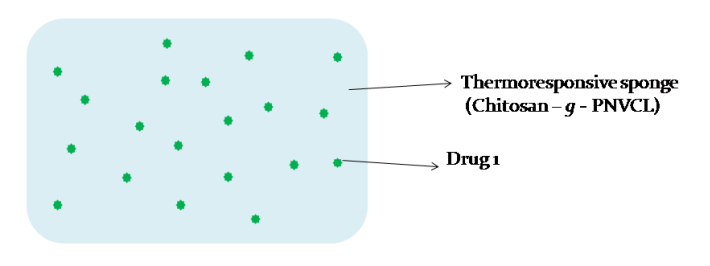
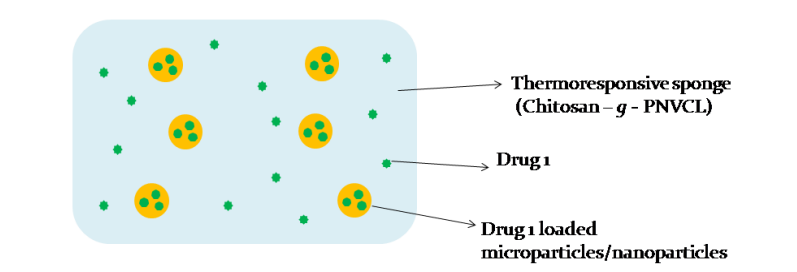
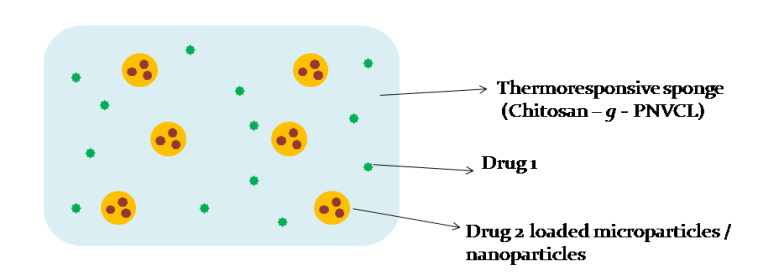
A smart, thermoresponsive drug delivery system based on PNVCL-chitosan gel/sponges enables heat-triggered drug release, improving local bioavailability and patient compliance in managing pain, infection, or inflammation. The delivery system is customizable, biocompatible, and suitable for topical/transdermal applications.
Figure (1) Schematic representation of Drug loaded Thermo responsive sponge / scaffold / patch for transdermal delivery; (2) Schematic representation of Drug loaded Microparticles / Nanoparticles incorporated Thermo responsive sponge / scaffold / patch for transdermal delivery; (3) Schematic representation of Drug loaded Microparticles / Nanoparticles incorporated in drug loaded Thermo responsive sponge / scaffold / patch for transdermal delivery
Current systemic and invasive methods of delivering drugs for chronic conditions (e.g., osteoarthritis) suffer from poor bioavailability, side effects, and high treatment costs. There is a need for a localized, controlled, and patient-friendly drug delivery platform.
- Biocompatibility: The chitosan-grafted PNVCL gels are highly biocompatible, showing over 90% cell viability and no signs of skin irritation in in vivo tests.
- Phase Transition at Therapeutic Range: The system has a tunable LCST between 32°C and 42°C, allowing drug release to be triggered by mild external heat, such as a heating pad.
- Pulsatile Release Capability: The technology enables reversible, on–off drug release cycles controlled by alternating exposure to heat, offering precise dosing based on need.
- Versatile Drug Incorporation: The gel or sponge can encapsulate both hydrophilic and hydrophobic drugs, as well as microparticles, nanoparticles, and live cells.
- Ease of Use: Designed for non-invasive application, the system can be used in gel or patch form and is easy for patients to self-administer.
The technology prepared CP gels and sponges with Etoricoxib or Acetaminophen. It validated in vitro release kinetics, swelling, LCST, SEM imaging, FTIR, and in vivo skin safety studies.
The technology is currently at the lab-scale stage.
4
This technology empowers patients with responsive, localized drug therapy. It reduces side effects, improves compliance, and enhances treatment efficacy-especially valuable in resource-limited or elderly patient settings.
- Biomedical Engineering: Used in developing smart, responsive materials for targeted and controlled drug delivery systems
- Healthcare Devices: Integrated into non-invasive patches or gels for patient-friendly pain and infection management
- Regenerative Medicine: Supports cell attachment and release, aiding tissue repair and engineering applications
- Pharma and Drug Delivery: Provides a platform for localized, heat-triggered drug release, improving efficacy and reducing side effects
- Geriatric and Chronic Care: Enables easy, self-administered treatment for elderly patients with chronic pain or inflammation
- Orthopedics and Rheumatology: Delivers anti-inflammatory drugs directly to joints, improving treatment for osteoarthritis and similar conditions
Geography of IP
Type of IP
3808/MUM/2014
366957