Thermal sorption studies on Mg(H2) for vehicular applications
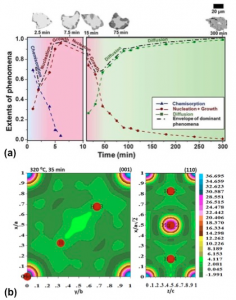
MgH 2 is a promising material for on-board vehicular hydrogen storage applications, due to its high gravimetric capacity (7.6 wt. %). If employed efficiently, it has the potential to be utilised in Proton Exchange Membrane (PEM) fuel cells, which can replace the conventional IC-engines driven by fossil fuels. However, its application is limited by its slow kinetics of hydrogen absorption and desorption collectively known as ‘sorption’.Hydrogen sorption mainly involves several kinetic phenomena Eg. chemisorption (H-atom dissociation/recombination), nucleation and growth of metal or hydride by interfacial movement (NG) and/or diffusion. Our rigorous experimental and mathematical analysis supported by electron microscopic observations suggests that multiple phenomena can simultaneously control the hydrogen absorption in Mg to varying extents with time (Fig. 1a). Hence, the rate controlling phenomenon can change with time, contrary to the popular belief in literature. During hydrogen desorption the charge density maps reveal that not all the hydrogen atoms are equivalent in their removal from the MgH 2 unit cell (Fig. 1b), hence can be individually affected by the transition states formed upon catalyst addition.
Electrochemical sorption studies on Pd(H x ) and Pt(H x ) for sensor applications
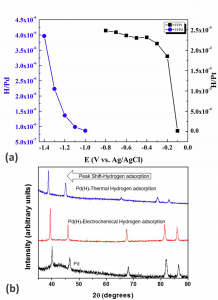
Pd and Pt are the benchmark materials for electrochemical hydrogen sensors. Electrochemical hydrogenation studies on Pd and Pt suggest hydrogen adsorption to varied extents and sensitivities at different potentials (Fig. 2a). The X-Ray diffraction (XRD) patterns of the Pd and electrochemically hydrogenated Pd demonstrate a significant peak shift to lower angles upon hydrogen adsorption. Such an indirect characterisation of hydrogen adsorption is affirmed by the XRD pattern of the thermally hydrogenated Pd (Fig. 2b).
Prof. Sankara Sarma V. Tatiparti
