This innovation is a simple, low-cost, and easy-to-use test system designed to help women detect ectopic pregnancy early. It uses urine test strips (like a pregnancy test) and a smartphone app to track pregnancy hormone levels over a few weeks. The app compares the hormone levels to normal patterns and alerts the user if something seems wrong, prompting early medical attention. It is specially designed to be used at home and is ideal for rural or low-resource areas where access to hospitals is limited.
Detecting ectopic pregnancy (when a fertilized egg grows outside the uterus) early is a serious medical challenge. Regular urine pregnancy tests can confirm pregnancy but cannot detect if it is ectopic. Without timely detection, this condition can lead to complications such as fallopian tube rupture and serious health risks for the mother. Currently, confirming ectopic pregnancy requires expensive and time-consuming blood tests or ultrasounds, which may not be easily accessible.
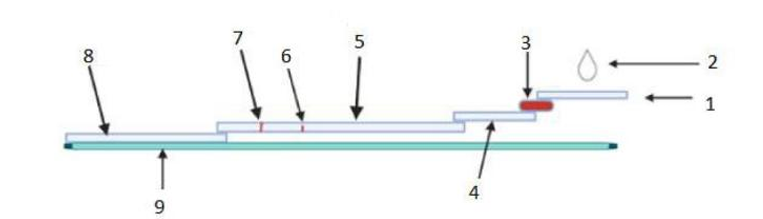
- At-Home Test Strip System: This product uses lateral flow test strips that work similar to standard pregnancy tests but are enhanced to track hormone levels over time.
- Smartphone App for Weekly Monitoring: This feature involves a mobile app that reads strip results using a smartphone camera and compares hormone levels across different weeks.
- Stacking Pad for Better Accuracy: This feature includes an extra layer inside the strip that allows more time for hormone detection, making the test more sensitive and reliable.
- Wide Range Hormone Detection: This product can measure very low to very high levels of pregnancy hormones (from 10 IU/L to 200,000 IU/L), making it suitable for early and later stages.
- Personalized Alerts and Guidance: This feature uses the app to recommend when to take the next test and alerts the user if the results suggest a possible ectopic pregnancy.
The prototype includes a pack of customized urine test strips and a fully functional mobile app. Each test strip is matched with a specific buffer for a particular week of pregnancy. After the user inputs the date of their last period, the app calculates the current gestational week and suggests the correct strip and buffer to use. The smartphone camera takes a picture of the strip after the test, and the app calculates the hormone level using image analysis. The user is then guided on the next testing date and alerted about any warning signs.
The prototype of this diagnostic kit is already developed. It includes a set of specialized test strips and an app that reads the test result using the phone’s camera. The app uses a smart algorithm to guide users, track hormone levels across weeks, and signal possible risks. The invention is currently at the proof-of-concept stage, with working samples and a functioning mobile app designed for early testing after a positive pregnancy result.
3
This solution holds significant value, especially in areas where access to hospitals and diagnostic labs is limited. Women can test themselves from home and receive early warnings about potentially dangerous pregnancies. Early detection of ectopic pregnancy can prevent life-threatening complications, reduce emergency surgeries, and improve maternal care. The technology also promotes awareness and self-monitoring, empowering women to seek help on time. It reduces the economic burden by avoiding repeated hospital visits, and its simplicity ensures it can be used even without professional assistance.
- Pregnancy health tracking and early risk detection
- Women’s health diagnostics
- Home-based medical testing kits
- Rural and low-resource healthcare delivery
- Digital health and mobile healthcare platforms
- Emergency obstetric care and maternal monitoring
Geography of IP
Type of IP
202321048341
559895