The invention discloses a low-coordinate Co(II) complex that catalyzes the selective conversion of CO₂ into methanol equivalents under mild conditions. The catalyst operates without additives such as Lewis acids or amines and shows high turnover numbers and long-term stability. It also tolerates common impurities like CO, SOx, and NOx, enabling direct application to flue gas streams. Comparable Cu(I) and Fe(II/III) analogues also demonstrate efficient performance, making the system suitable for practical CO₂ valorization.
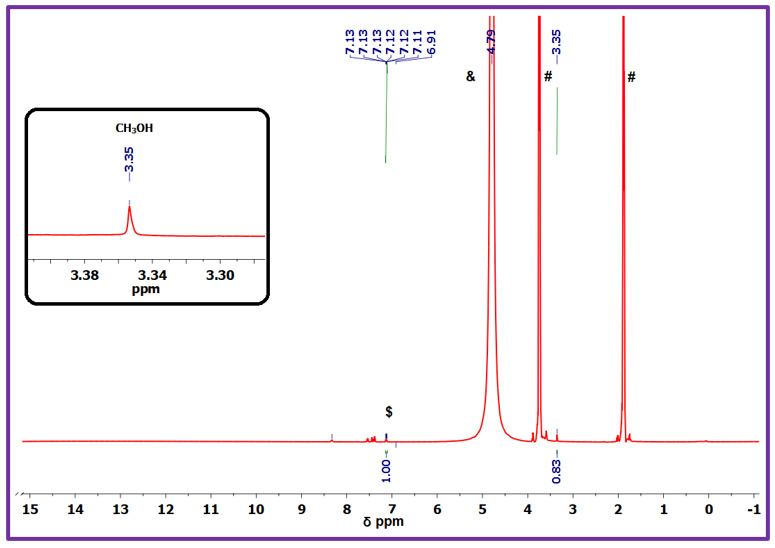
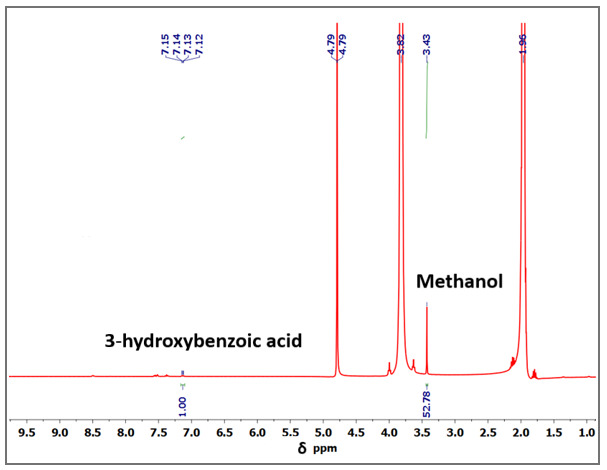
Figure (1) 1H-NMR spectra showing CO2 conversion into CH3OH using a copper dimer catalyst at room temperature where $ = 3-hydroxy benzoic acid, &= D2O, # = THF; (2) A representative 1H-NMR spectrum (S.No.3) showing the formation of methanol recorded in a 400 MHz instrument at room temperature; (3) Top: Capturing of the vehicle exhaust into the gas bladder, Bottom: General scheme showing the CO2 (Vehicle exhaust) reduction to methanol using 1
Existing methods for CO₂ hydrogenation into methanol rely heavily on high temperatures, high pressures, toxic additives, and expensive noble metal catalysts, which limit their practical and sustainable application. Many 3d transition metal catalysts suffer from low efficiency, catalyst poisoning, and loss of activity due to product or co-reactant interference. There is a critical need for a robust, low-coordinate, additive-free Co(II)-based catalytic system that can efficiently convert CO₂ to methanol equivalents under mild, scalable, and environmentally friendly conditions.
- Low-Coordinate Co(II) Catalyst: The invention provides a Co(II) complex with a coordination number of three or less. This design offers an open site that facilitates direct CO₂ binding and activation.
- Additive-Free Reaction Conditions: The catalyst system operates without requiring Lewis acids, amines, or other additives. This eliminates catalyst poisoning and simplifies the overall reaction environment.
- High Catalytic Efficiency: The Co(II) complex achieves a turnover number of 11,171 and TOF of 508 h⁻¹. It shows higher efficiency than other reported 3d transition metal-based catalysts.
- Mild Reaction Conditions: The catalytic process occurs between 25°C–60°C and under 0.1–1 bar CO₂ pressure. These mild conditions minimize energy use and reduce system complexity.
- Versatility across Metal Centers: The same catalytic strategy has been demonstrated with Cu(I) and Fe(II/III) complexes. These analogues show high TONs, reaching up to across multiple cycles.
The prototype was developed using a low-coordinate Co(II) complex synthesized by reacting lithium anilide with CoBr₂ in dry THF under inert conditions. For catalytic testing, 2.5 mg of the Co(II) complex and 328 mg of sodium borohydride were combined in dry THF and exposed to CO₂ at 0.1 bar pressure and 60°C for 22 hours. The reaction resulted in the formation of methanol equivalents, confirmed by ¹H NMR spectroscopy through the detection of trimethoxyborane. Similar catalytic tests using Cu(I) and Fe(II/III) analogues were also performed, demonstrating comparable efficiency and stability.
The catalytic system has been successfully demonstrated at the laboratory scale using low-coordinate Co(II), Cu(I), and Fe(II/III) complexes. Repeated experimental cycles confirmed stable conversion of CO₂ to methanol equivalents with high turnover numbers and no catalyst deactivation. The catalyst retains performance across multiple runs, and the system has been validated under mild pressures (0.1–1 bar CO₂) and temperatures (25°C–60°C), proving its reproducibility, efficiency, and potential for scale-up.
5
This invention offers a sustainable and energy-efficient approach for converting CO₂ – a major greenhouse gas – into methanol, a valuable fuel and industrial feedstock. By using a low-cost, additive-free Co(II) catalyst under mild conditions, the technology supports carbon recycling and addresses climate change mitigation. Its compatibility with flue gas components makes it highly relevant for industrial CO₂ utilization and emission reduction strategies.
- Clean energy and carbon capture: The technology efficiently converts CO₂ into methanol equivalents under mild conditions, supporting sustainable energy and carbon mitigation strategies.
- CO₂ valorization technologies: It enables selective reduction of atmospheric or anthropogenic CO₂ sources (e.g., flue gas, vehicle exhaust) into valuable methanol derivatives without catalyst deactivation.
- Methanol fuel production: The produced trimethoxyborane can be hydrolyzed into methanol, making it relevant for synthetic fuel generation from CO₂.
- Greenhouse gas mitigation: It offers a scalable chemical approach to reduce the CO₂ burden from industrial emissions by transforming it into usable products.
- Chemical and fuel industries: It is applicable in methanol synthesis, catalysis, and feedstock conversion using low-cost and stable cobalt-based systems.
- Automotive and industrial exhaust treatment: It has demonstrated capability to selectively reduce CO₂ from vehicle exhaust and similar gas streams containing multiple pollutants.
Geography of IP
Type of IP
202321035458
560409