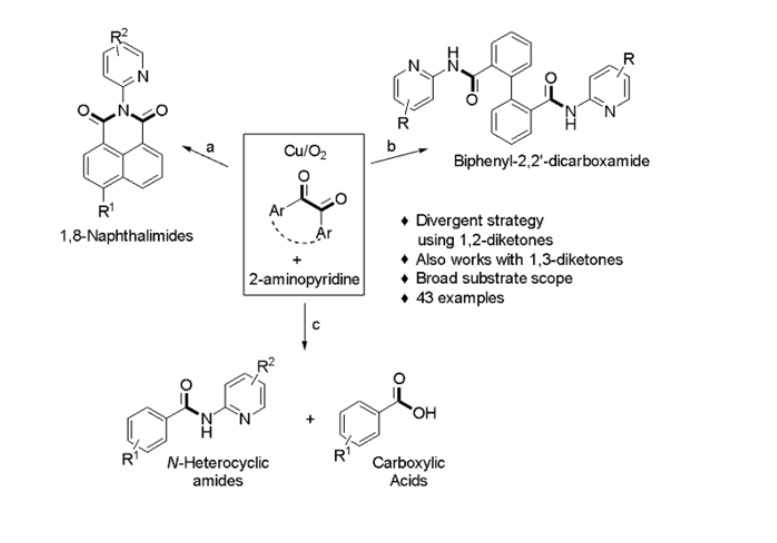
The invention provides a copper-catalyzed process for synthesizing 1,8-naphthalimide, biphenyl dicarboxamide, and N-heterocyclic amide derivatives from 1,2-diketones and 2-aminopyridines. The method involves C–C bond functionalization under aerobic conditions using CuCl2 and additives such as pyridine or pivalic acid. It enables the formation of structurally diverse amides in good to excellent yields, under mild and scalable conditions, without the need for pre-functionalized starting materials.
Conventional methods for synthesizing 1,8-naphthalimides, biphenyl dicarboxamides, and N-heterocyclic amides often involve multistep procedures, pre-functionalized reagents, and harsh conditions that limit substrate scope and functional group tolerance. There is a need for a one-step, efficient, and modular process that enables direct transformation of readily available 1,2-diketones into diverse amide derivatives using simple catalytic systems under mild, aerobic conditions.
- Copper-Catalyzed C-C Bond Activation: The invention uses a CuCl2-catalyzed oxidative strategy to cleave C–C bonds in 1,2-diketones. This enables direct synthesis of amide frameworks under aerobic, metal-mediated conditions.
- Broad Substrate Scope: The process allows conversion of various 1,2-diketones and 2-aminopyridines into structurally distinct amides. Both electron-donating and -withdrawing substituents are well tolerated.
- Single-Step Synthesis: The reaction proceeds in one pot without needing pre-functionalized reagents or protective groups. This increases atom economy and simplifies purification.
- Mild and Scalable Conditions: The method operates between 70°C and 100°C using air or oxygen as the oxidant. It is compatible with scalable solvents like t-BuOH, acetonitrile, or m-xylene.
- Versatile Product Formation: The process yields 1,8-naphthalimides, biphenyl dicarboxamides, and N-heterocyclic amides. These products exhibit properties useful in optoelectronics, dyes, and bioactive materials.
The prototype was developed using a copper-catalyzed reaction between 1,2-diketones (such as acenaphthoquinone or phenanthrenequinone) and 2-aminopyridine derivatives. The optimized procedure involved heating the substrates in the presence of CuCl2 (1.5 equivalents) and pyridine or pivalic acid in solvents like t-BuOH, m-xylene, or acetonitrile at 70°C–100°C under an oxygen or air atmosphere. The reaction yielded various amide derivatives in 50%–98% yields. The products were purified by column chromatography and structurally confirmed by NMR, HRMS, IR, and in some cases by single-crystal X-ray diffraction. The reaction conditions were successfully applied to over 25 substrate combinations, demonstrating reproducibility and scalability.
The process has been successfully demonstrated at laboratory scale across a diverse set of substrates, including acenaphthoquinone, phenanthrenequinone, and substituted aminopyridines. Multiple derivatives of naphthalimides, biphenyl dicarboxamides, and N-heterocyclic amides have been synthesized in yields up to 98%. The reaction conditions have been optimized for temperature, solvent, and catalyst loading, and several products were confirmed by spectroscopic analysis and X-ray crystallography, validating structural integrity and scalability.
5
The invention provides a direct and efficient route to synthesize valuable amide-based compounds used in pharmaceuticals, organic semiconductors, agrochemicals, and fluorescent materials. By eliminating the need for pre-functionalized starting materials and reducing process steps, the technology supports greener and more cost-effective chemical manufacturing. Its broad substrate compatibility and operational simplicity make it suitable for scalable industrial and academic use, thereby contributing to sustainable development in materials and medicinal chemistry.
- Pharmaceutical intermediate synthesis
- Organic electronic and semiconductor materials
- Fluorescent dye and imaging agent development
- Agrochemical compound synthesis
- Functional materials and optoelectronic devices
- Academic and industrial organic synthesis research
Geography of IP
Type of IP
201821028477
399734