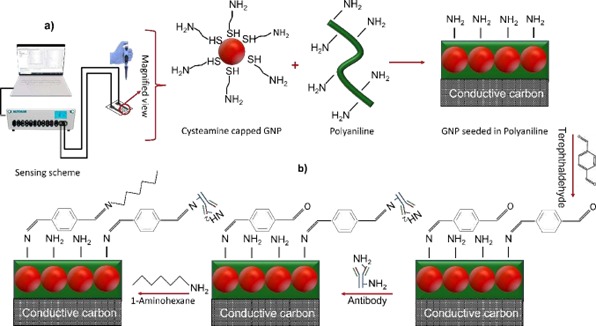
This invention describes an electrochemical biosensor for detection of biological toxins. Embodiments herein further disclose a method of manufacturing the electrochemical biosensor and a method for detection of biological toxins using the electrochemical biosensor. The electrochemical biosensor, according to embodiments herein includes a carbon electrode embedded with a composite comprising at least one conductive polymer and at least one nanoparticle functionalized with primary amine; at least one bidirectional cross-linker; and at least one blocking agent. Further the biosensor can be used in impedance spectroscopy as well as in a voltammetry based 3 electrode system. The biosensor is portable, simple to use, cost-effective, having high sensitivity and easy to manufacture.
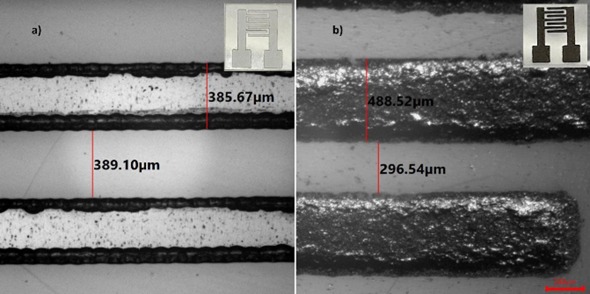
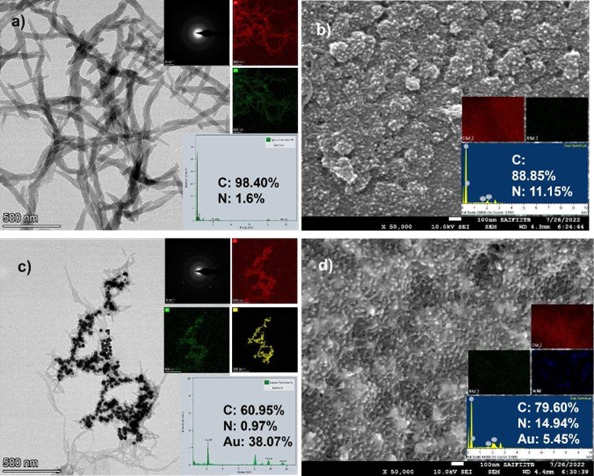
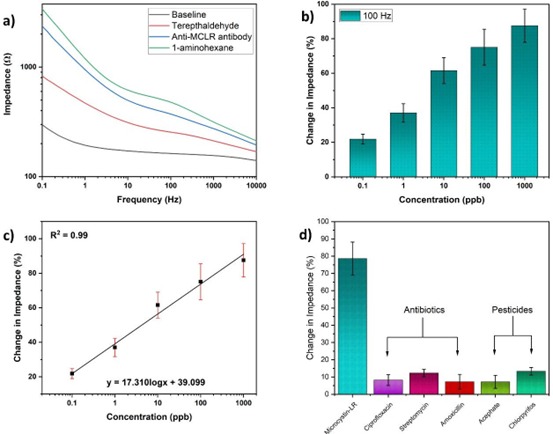
Figure (1) Schematic of experimental setup and sensor preparation, (1a) Experimental setup using SPCE substrate, (1b) Substrate preparation and immobilization process for immunosensing; (2) Stencil printed carbon interdigitated microelectrode, (2a) Optical microscope image of stencil fabricated on PET sheet. Inset: Image of stencil on PET sheet, (2b) Optical microscope image of carbon electrodes on PET sheet; Inset: Image of electrodes on PET sheet; (3) Electron microscopic images of SPCE substrate, (3a) Field Emission Gun TEM (FEGTEM) micrograph of polyaniline which forms a thread like structure after polymerisation, (3a Inset) Diffraction pattern denoting amorphous nature, (3b) FEGSEM micrograph of polyaniline deposited over the carbon electrodes. The EDAX mapping for image set a and b shows the presence of nitrogen (N), indicating polyaniline, which has amines, (3c) TEM image of CGNPs embedded in polyaniline, (3c inset) Diffraction pattern indicating the crystalline nature, (3d) Image of the carbon electrode surface when CGNPs embedded within polyaniline were deposited over it. The EDAX mapping for image set c and d shows the presence of nitrogen (N) and gold (Au), indicating polyaniline and gold nanoparticles; (4) Initial studies done on potentiostat, (4a) Impedance change during the functionalisation steps of the substrate, (4b) Relative change in impedance with respect to baseline for Microcystin-LR (MCLR) in PBS, (4c) Calibration curve generated for MCLR in PBS, (4d) Responses for different interfering species for 1 ppm of concentration.
Biological toxins such as Microcystins, botulinum, and aflatoxins pose severe threats to human health and water safety. Existing detection methods are often expensive, time-consuming, require complex sample processing, and lack portability for on-site testing.
- Advanced Electrode Design: Uses stencil-printed carbon interdigitated microelectrodes (SPCE) for high surface area and miniaturization.
- Novel Composite Material: Incorporates polyaniline and cysteamine-capped gold nanoparticles to enhance conductivity and sensor sensitivity.
- Sensitive Detection Mechanism: Employs electrochemical impedance spectroscopy (EIS) for accurate detection of microcystin-LR in the ppb range.
- Field Deployable and Scalable: Low-cost fabrication and portable design make it suitable for on-site environmental monitoring.
- Rapid Functionalization: Sensor assembly after composite drying takes less than 3 hours, allowing quick deployment.
- Versatile Sample Compatibility: Capable of analyzing water and biological fluids, making it applicable in diverse testing environments.
The prototype consists of a stencil-printed carbon interdigitated microelectrode (SPCE) designed with electrode finger widths ranging from 465 to 505 μm and inter-finger gaps between 280 to 320 μm. The electrode is modified with a nanocomposite comprising polyaniline and cysteamine-capped gold nanoparticles (CGNPs), which enhances conductivity and facilitates biomolecule immobilization. The composite layer is air-dried at room temperature over four days to ensure stability and uniform deposition. Following this, the sensor is functionalized with a bidirectional cross- linker, anti-Microcystin-LR antibodies, and a blocking agent in a process completed within three hours. Detection is achieved through electrochemical impedance spectroscopy (EIS), enabling sensitive, label-free analysis of biological toxins.
The technology has been demonstrated and/or validated in lab.
4
The electrochemical biosensor offers significant societal impact by enabling real-time, on-site detection of biological toxins such as Microcystin-LR in drinking water sources, helping to safeguard public health. Its portability and rapid response support early warning systems for toxin-related outbreaks and monitoring of harmful algal blooms. The low-cost, easy-to-use design makes it suitable for resource-limited settings, with potential for integration into point-of-care diagnostic tools.
- Environmental monitoring and water quality assessment
- Healthcare and medical diagnostics
- Food and beverage safety
- Biosensor manufacturing and point-of-care devices
- Wastewater treatment plants and public health agencies
Geography of IP
Type of IP
202421030360
562042