The present invention provides a point-of-care device for the rapid and reliable detection of Vitamin B12 deficiency using a competitive lateral flow immunoassay. The device comprises an immunochromatographic test strip integrated with an Internet of Things (IoT) module for real-time data capture, analysis, and communication. Designed for affordability, sensitivity, and ease of use, the system enables faster diagnosis at or near the patient site, eliminating the need for centralized laboratory infrastructure.
Current methods for detecting Vitamin B12 deficiency are time-consuming, costly, and require centralized laboratory infrastructure, leading to delays in diagnosis and treatment. Existing POCT devices lack specificity and optimization for Vitamin B12 detection. Therefore, there is a need for a rapid, cost-effective, user-friendly point-of-care device specifically designed for accurate Vitamin B12 quantification.
- Shunt Pad-Enhanced Sensitivity for Ultra-Low Detection: The integration of a hydrophilic shunt pad delays flow, increasing interaction time between sample and antibody, allowing high sensitivity detection of vitamin B12 in physiological (pmol/L) range—a critical feature for accurate diagnosis of subclinical deficiency.
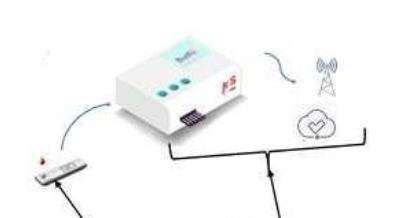
- IoT-Enabled Smart Connectivity with Cloud Integration: The device is equipped with microcontrollers, sensors, and a Wi-Fi module, the device supports real-time data upload, remote access, and cloud storage, turning a traditional strip test into a smart connected diagnostic tool—ideal for hospitals, telemedicine, and decentralized care.
- Rapid and Portable Point-of-Care Design (ASSURED-compliant): The lateral flow format ensures rapid results without laboratory infrastructure. It aligns with WHO’s ASSURED criteria (Affordable, Sensitive, Specific, User-friendly, Rapid/Robust, Equipment-free, and delivered), making it perfect for rural, mobile, and bedside diagnostics.
- Gold Nanoparticle-Based Competitive Immunoassay: It utilizes gold nanoparticle-conjugated monoclonal antibodies, offering a visually interpretable signal and enabling semi-quantitative estimation. Commercially, this ensures both ease of use and reliable results without needing advanced readers.
- Optimized B12-BSA Hapten Chemistry for Stability and Accuracy: It employs EDC/NHS chemistry to stably bind monocarboxylate B12 to BSA, ensuring strong, specific immobilization on the test line and high signal reproducibility, a key for regulatory and commercial validation.
- Cost-Effective, Scalable Manufacturing: It avoids complex or hazardous methods (no cyanide, no ELISA), instead using readily available reagents (e.g., citrate, BSA, HAuCl4) and paper-based materials, making the device easy to mass-produce, affordable, and attractive to healthcare providers.
The prototype of the present invention is a compact, handheld point-of-care device for rapid detection of vitamin B12 using a competitive lateral flow immunoassay strip integrated with an IoT-enabled reader. The test strip includes a sample pad, conjugate pad with antibody-gold nanoparticle conjugate, shunt pad for enhanced sensitivity, a nitrocellulose reaction membrane with test and control lines, and an absorbent pad on a plastic backing. A key innovation is the shunt pad, which slows fluid flow to boost antigen-antibody interaction, enabling accurate detection in the pmol/L range. The reader is embedded with microcontrollers, sensors, and a Wi-Fi module for quantifying signal intensity and storing results to the cloud. The prototype supports real-time result sharing, remote monitoring, and is compliant with ASSURED criteria, making it ideal for decentralized diagnostics.
The technology is currently at TRL 5 - validated in Lab environment with real sample.
5
This point-of-care device enables rapid, accurate, and affordable detection of vitamin B12 deficiency, especially in remote and underserved areas. It reduces dependence on centralized labs and long turnaround times, promoting timely diagnosis and treatment. Its portability and IoT connectivity support better patient monitoring and data sharing. The device is particularly impactful for maternal health, geriatric care, and malnutrition surveillance.
- Point-of-care diagnostics
- Clinical biochemistry and pathology
- Nutritional health monitoring
- Medical devices and diagnostics
- Rural and primary healthcare
- Biomedical sensor technology and IoT integration
Geography of IP
Type of IP
202021008083
513539