The present invention relates to a simple, effective, and economical autonomous wearable device for transdermal drug delivery. It comprises a transdermal patch embedded with a hollow out-of-plane silicon microneedle array integrated with a drug reservoir. The microneedles enable precise and minimally invasive delivery of therapeutically effective doses of anti-emetic drugs for efficient management of Chemotherapy-Induced Nausea and Vomiting (CINV). The invention also includes the design and development of embedded electronics for autonomous operation of the device. Furthermore, the method for fabrication of the microneedle array and assembly of the wearable device is disclosed. While primarily intended for CINV, the device can be extended to deliver drugs for various chronic and acute medical conditions.
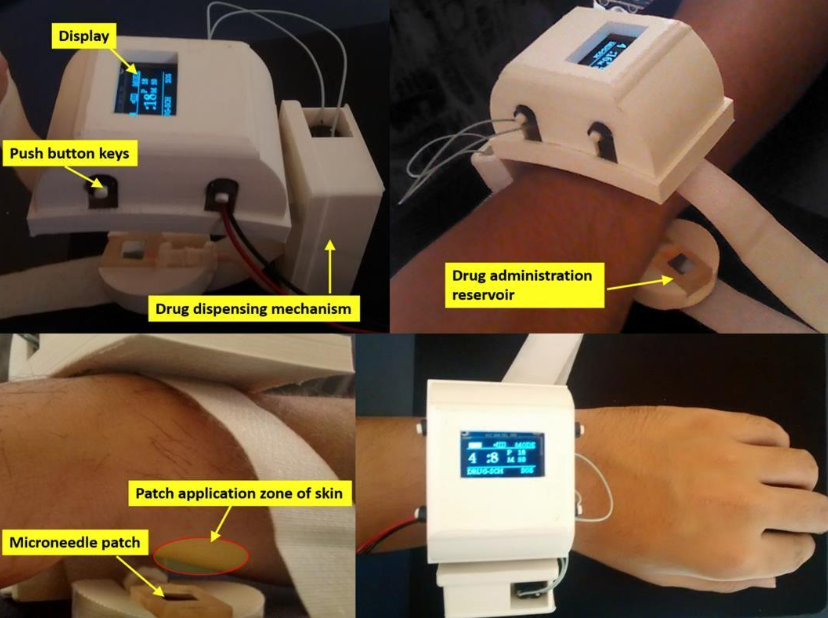
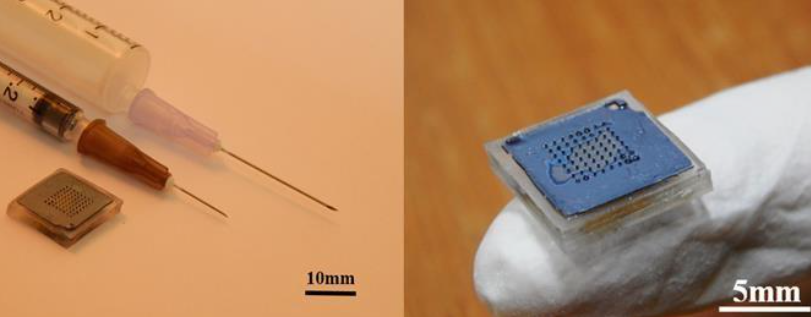
Figure (1) Representative images of the finished working prototype worn on the human wrist - Display screen, drug dispensing mechanism, drug administration reservoir, microneedle patch, patch application zone on ventral side of wrist is marked in the representative images; (2) Representative image of minimally invasive microneedle patch for painless drug delivery - the microneedle patch is integrated with a wearable device as shown in figure 1.
Chemotherapy-Induced Nausea and Vomiting (CINV) is a severe and unpredictable side effect of cancer treatment that can lead to serious complications such as dehydration, electrolyte imbalance, nutritional deficiency, and even withdrawal from therapy.
Existing drug delivery methods—oral, intravenous, and intramuscular—suffer from major drawbacks including pain, poor compliance, high cost, low bioavailability, and the need for trained personnel. While transdermal microneedle-based delivery offers a promising alternative, current systems are limited by complex applicators and high cost. There is an urgent need for a simple, economical, autonomous, and patient-friendly transdermal drug delivery system that effectively manages CINV and improves patient quality of life.
- Painless Transdermal Drug Delivery: The invention uses bioinspired microneedles to provide a pain-free alternative to injections, improving patient comfort and compliance during treatment.
- Enhanced Drug Permeation and Skin Penetration: Ex-vivo studies demonstrate up to 5,000-fold increase in drug permeation and penetration depth of ~400 µm, ensuring efficient transdermal delivery of antiemetics.
- Proven Biocompatibility: In-vitro testing with human keratinocyte cell lines shows over 95% cell viability, confirming that the microneedle material is safe for long-term use.
- Regulatory-Compliant Wearable Prototype: A fully functional wearable device has been developed according to FDA standards, incorporating human factor design for real-world clinical usability.
- Integrated Embedded System: The prototype features built-in electronics, user interface, firmware, and peristaltic actuators, enabling autonomous and programmable drug delivery.
- Improved Clinical Outcomes and Quality of Life: The system supports continuous, controlled antiemetic therapy, helping to reduce chemotherapy side effects and enhance overall patient well-being.
- Applicability beyond CINV: While designed for chemotherapy-induced nausea and vomiting, the technology can be extended to other chronic and acute conditions requiring controlled drug delivery.
The prototype consists of a bioinspired hollow silicon microneedle patch integrated with a drug reservoir, designed in an out-of-plane array for efficient and painless transdermal drug delivery. Each microneedle features a cylindrical body with a tip, a base, and a through-channel for drug flow, with dimensions of ~250–300 µm in length and ~60–80 µm in outer diameter. The patch demonstrates structural stability, high drug permeation (up to 5×10³ fold), and biocompatibility as confirmed through in-vivo studies. The microneedle patch is fabricated using advanced microfabrication techniques like photolithography, RIE/DRIE, and Argon milling, while the drug reservoir is created from PDMS using soft lithography. The patch is integrated into a fully autonomous wearable drug delivery device, which includes embedded electronics, a microcontroller with configurable firmware, a peristaltic actuator, and a user interface with OLED display. The device supports personalized dosing regimens and is ergonomically designed considering human factor engineering, allowing lay users to operate it safely at home or outdoors without medical supervision.
At TRL 4, the wearable, autonomous drug delivery device has been validated in a lab setting. A functional prototype integrates hardware, configurable firmware, actuators, minimally invasive microneedle module, and personalized dosing algorithms, with in-vitro testing confirming precise drug release profiles for chronic disease management. Core safety and control systems are bench-validated, and biocompatibility has been demonstrated in a preclinical model. The device design incorporates human factors engineering aligned with medical device standards to ensure usability and patient adherence. The technology is now ready for TRL 5 validation and strategic licensing for clinical development/ manufacturing/ translation.
4
This invention offers a patient-friendly, pain-free solution for managing chemotherapy-induced nausea and vomiting, significantly improving treatment adherence and comfort. Its biocompatibility and ease of use make it suitable for home-based care, especially for vulnerable patients. Overall, it enhances quality of life and promotes accessible, affordable healthcare for chronic disease management.
- Oncology and supportive cancer care
- Transdermal drug delivery systems
- Biomedical device engineering
- Embedded medical electronics
- Chronic Disease Management Systems
Geography of IP
Type of IP
201721020761
450160