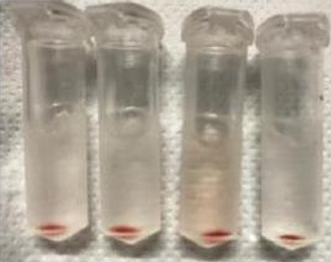
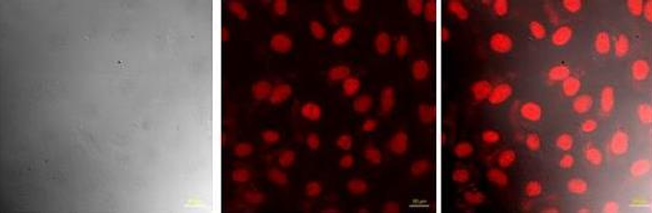
The present invention provides drug loaded nanohybrids. Particularly, the present invention provides drug loaded erythrocytes (red blood cells) as nanohybrids for drug delivery and imaging applications. The erythrocytes may also be loaded with imaging dyes or probes. The erythrocytes act as nano-carriers and they have higher biocompatibility, less toxicity and higher theranostics efficacy. The present invention also provides a process for preparing the drug loaded nanohybrids.
Current nanoparticle-based drug delivery systems, though promising, face significant limitations such as high production costs, short half-life, potential toxicity, and limited biocompatibility. Liposome and polymer-based nanocarriers often rely on synthetic materials, leading to moderate stability and undesirable side effects. There is a critical need for a biocompatible, non-toxic, and efficient nano-delivery platform that offers high drug loading capacity, prolonged circulation, and targeted delivery to tumor sites while minimizing harm to healthy tissues.
- Completely Biocompatible: Since the nanohybrid is derived from red blood cells (RBCs), these nanohybrids are naturally safe for biological use, reducing toxicity and immune response.
- Natural Targeting and Cell Binding Ability: The Inherent biological properties of RBCs allow the particles to selectively bind to cancer cells, improving targeted drug delivery and minimizing off-target effects.
- Large Cargo Capacity: The spacious inner cavity enables high loading of drugs (like doxorubicin), dyes, or imaging probes, reducing dosing frequency and enhancing therapeutic payload.
- High Stability and Uniform Particle Size: The nanoparticles maintain consistent size and structure, ensuring predictable in vivo behaviour and performance, verified through DLS and TEM.
- Localized Imaging with Cancer Cells: Natural targeting allows precise accumulation at tumor sites, enabling combined diagnostic imaging and therapy (theragnostic).
- Simple, Ambient-Condition Preparation: Nanoparticles are prepared easily under room temperature without toxic chemicals, making the process safe and cost-effective.
- Reproducible and Scalable Synthesis in Aqueous Medium: The water-based process is environmentally friendly and suitable for industrial-scale production.
- Highly Dispersible and Stable in Physiological Conditions: Nanohybrids remain stable and well-dispersed in body fluids, preventing aggregation and ensuring effective circulation and delivery.
The method for producing drug-loaded nanohybrids begins by providing anucleated red blood cells, which are first treated with a buffered saline solution to maintain membrane stability. This is followed by a hypotonic treatment that lyses the cells and removes their internal contents, effectively converting them into empty membrane shells. These treated red blood cells are then concentrated to form white- colored nanoparticles. In the next step, a drug, imaging probe, or dye is added to the nanoparticle suspension, and the mixture is subjected to sonication to facilitate efficient loading of the therapeutic or diagnostic agents into the nanoparticle cavities. Finally, the solution is further concentrated to yield the drug-loaded nanohybrids, which are then ready for use in targeted drug delivery and biomedical imaging applications.
This has been developed at the laboratory scale and tested in vitro.
3
This invention offers a highly biocompatible and safe platform for targeted drug delivery, significantly reducing side effects associated with conventional cancer treatments. By using red blood cell-derived nanocarriers, it promotes sustainable and non-toxic therapeutic solutions. The water-based production process is environmentally friendly and suitable for industrial-scale production.
- Targeted cancer therapy
- Molecular imaging and Theranostics
- Nanomedicine and bioengineering
- Hematology and blood-derived therapeutics
- Pharmaceutical drug delivery systems
- Personalized and precision medicine
- Biomedical device and implant coatings
- Regenerative medicine and cellular therapy
Geography of IP
Type of IP
202121012660
446412