A polymer-based drug delivery system utilizing a chitosan sponge matrix embedded with drug-loaded polymeric microparticles. This biodegradable system offers sustained, localized release of antibiotics for the treatment and prevention of implant-related infections.
Implant-related and surgical site infections are often treated with systemic antibiotics or antibiotic-loaded bone cements, both of which have limitations like burst drug release, incomplete infection control, antibiotic resistance, and the need for surgical removal.
- Localized Delivery: The system delivers drugs directly to the infected implant or wound site, ensuring higher local drug concentration and enhanced therapeutic efficacy.
- Controlled & Sustained Release: It provides a consistent and prolonged drug release over a period of 4 to 5 weeks, reducing the need for frequent dosing.
- Versatile Drug Compatibility: The delivery platform is compatible with a wide range of antibiotics, including both hydrophilic and hydrophobic drugs.
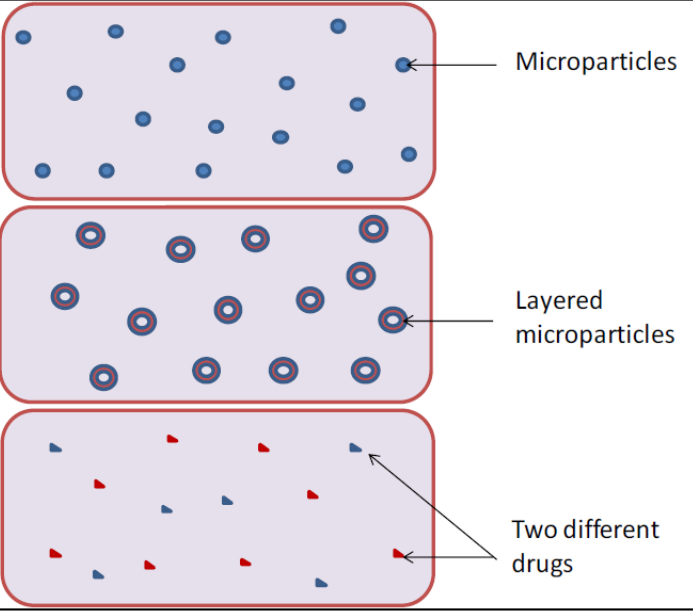
- Microparticle Embedding: Incorporating drug-loaded polymeric microparticles within the chitosan matrix helps minimize burst release and enhances drug bioavailability at the target site.
- Crosslinkable Matrix: The chitosan matrix can be chemically crosslinked to fine-tune its porosity, allowing precise control over the drug release kinetics.
- Non-surgical Removal: The system is fully biodegradable and biocompatible, eliminating the need for surgical removal after treatment.
- Scaffold for Healing: In addition to drug delivery, the porous structure of the matrix supports cell attachment, growth, and proliferation, promoting tissue regeneration and wound healing.
It is developed as a porous chitosan sponge containing microparticles of drugs like ciprofloxacin, vancomycin, and cefuroxime. Prototype validated using SEM, FTIR, in vitro drug release, antibacterial activity, and MTT assay for biocompatibility.
This technology is prototype ready.
4
It reduces treatment costs, minimizes antibiotic resistance risks, and supports faster recovery in orthopedic and post-surgical care through localized therapy. It eliminates repeated hospitalization or surgeries for infection control.
- Biomedical Implants: Infection prevention and treatment for implanted devices
- Surgical Wound Management: Localized antibiotic delivery and healing support
- Orthopedic Infection Treatment: Long-term drug release for bone/joint infections
- Hospitals and Surgical Centers: Post-operative infection control solution
- Controlled Drug Delivery Systems: Platform for localized and sustained drug release
Geography of IP
Type of IP
3278/MUM/2014
374364