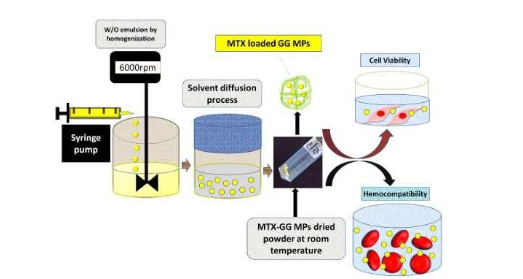
The present invention relates to a simple, economical process for synthesizing methotrexate loaded gellan gum microparticles (MTX-GG MPs) by a surfactant-free, water-in-oil (W/O) emulsion solvent diffusion method. The MTX-GG MPs on further characterization show enhanced encapsulation efficiency, drug loading capacity, drug release profile, physicochemical, biocompatibility and thermal stability properties. These microparticles find application as in vivo drug delivery agents for treating cancer and rheumatoid arthritis. This patent involves detailed formulation preparation and physicochemical characterizations.
Despite the therapeutic potential of Methotrexate (MTX), its poor water solubility, short half-life, and systemic toxicity limit its clinical effectiveness. Existing gellan gum (GG) microparticle fabrication methods exist to microencapsulate MTX but they either produce large particles or require costly equipment. There remains a need for a simple, economical, and surfactant-free method to develop small-sized MTX-loaded GG having high encapsulation efficiency and loading capacity.
- Enhanced Hydrophobic Drug Loading: The process overcomes the drawback of insufficient loading of hydrophobic drugs in the polymeric implant or hydrogels due to drug aggregation/crystal formation.
- High Loading Efficiency: It provides efficient loading of hydrophobic model drug methotrexate in the hydrophilic GG microparticles.
- Significantly Reduced Particle Size: The mean size of microparticles obtained is significantly less (4.54 µm) compared to the conventional processes where the size of microparticles were reportedly not less than 25 µm.
- Biocompatibility and Injectable Formulation: It develops MTX-GG MPs which are biocompatible and thus can be delivered directly to the target site via parenteral routes. Thus, it is advantageous over drug implants, since incorporation of implants into the patient’s body involve the risk of a surgical procedure.
The process begins by dissolving gellan gum (GG) in water through continuous stirring at a temperature of 80°C–90°C to form a GG polymer solution. Separately, methotrexate (MTX) is dissolved in an appropriate solvent. The MTX solution is then added to the GG polymer solution so that the final MTX concentration ranges between 0.025%–0.1% w/v in a 10 mL volume of the drug-polymer mixture. This solution is subsequently cooled and slowly added at a rate of 0.1–0.7 mL/min into 150–200 mL of ethyl acetate to form a water-in-oil (W/O) emulsion. The emulsion is simultaneously homogenized at about 5000–7000 rpm for approximately 6 minutes. After homogenization, the emulsion is covered and subjected to magnetic stirring at about 700–900 rpm for 1–3 hours to allow the solvent diffusion process to occur. The resulting GG-MTX microparticles are collected by centrifugation at about 8000–15000 rpm for 10–15 minutes and then dried to obtain pellet microparticles.
These technologies are available to be licensed to industry partners or collaboration for further development.
3
The invention enables safer and more effective delivery of methotrexate, improving treatment outcomes for cancer and autoimmune diseases. It reduces the need for invasive surgical implants, enhancing patient comfort and compliance. It offers a cost-effective drug delivery solution using biocompatible materials, making advanced therapies more accessible.
- Pharmaceutical industry
- Biotechnology industry
- Healthcare industry
- Medical devices industry
- Polymer formulation
Geography of IP
Type of IP
201721025703
400704