This invention introduces a biodegradable, biocompatible, polymeric film-based implant made from chitosan and polyvinyl alcohol (PVA), crosslinked with sodium tripolyphosphate (TPP), and loaded with the drug gemcitabine. The film is designed for local implantation near the pancreas, allowing for sustained and controlled release of GEM over a month. The film ensures high drug concentration at the tumor site, minimized systemic exposure, and improved treatment outcomes without harming healthy cells. It can also be adapted for other hydrophilic and hydrophobic anticancer drugs.
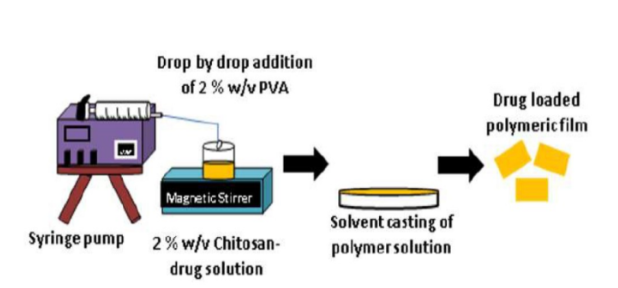
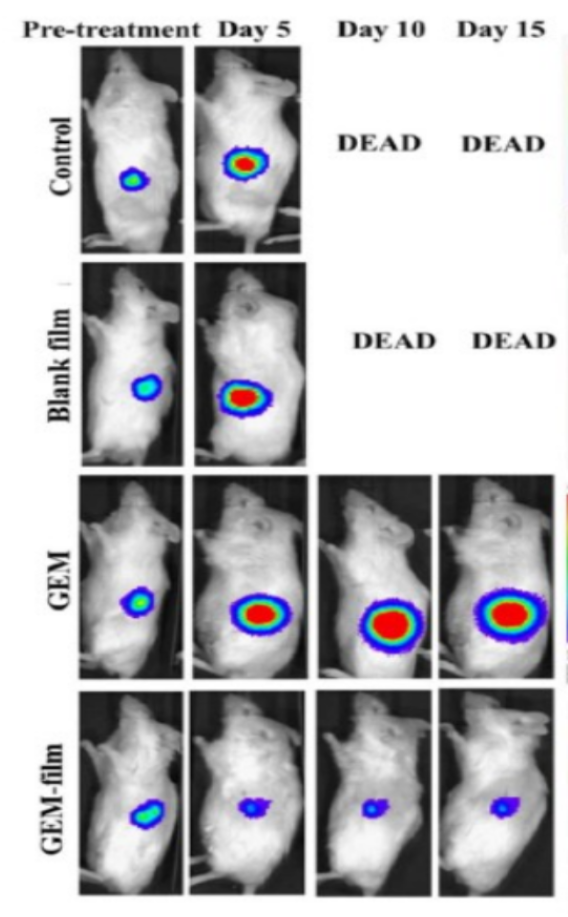
Figure (1) The gemcitabine-loaded chitosan-PVA composite film implant prepared using the solvent casting method; (2) The chitosan-PVA composite film-based implant developed for the localized and sustained delivery of gemcitabine; (3) Representative bioluminescence images depict tumor growth in a MiaPaca-2 orthotopic mouse model following treatment with blank film, free GEM, and GEM-loaded film.
Pancreatic cancer is difficult to treat due to late diagnosis, poor drug delivery to tumors, and drug resistance. The commonly used drug, GEM, has low stability in blood and causes side effects when given systemically. There is a need for a local, sustained delivery method that targets tumors directly while reducing harm to healthy cells.
- Chitosan-PVA Composite Structure: The film is made from a blend of natural chitosan and synthetic PVA, combining the biocompatibility and biodegradability with the mechanical strength. This allows for effective drug encapsulation.
- TPP-Based Crosslinking: Sodium tripolyphosphate (TPP) is used as a crosslinker to strengthen the film structure. It interacts with chitosan to create a stable network that enables the controlled and sustained delivery of GEM over several days without compromising biocompatibility.
- Hydrophilic and Hydrophobic Drug Compatibility: The composite film is capable of loading and releasing both hydrophilic drugs like GEM and hydrophobic agents such as curcumin. This makes the system adaptable for various drug types and treatment strategies targeting different cancers.
- Core-Enriched Drug Distribution: The drug is distributed in a gradient manner, with the highest concentration in the middle layer of the film. This structure enables gradual outward diffusion, maintaining consistent drug release and allowing for surface replenishment over time, extending therapeutic action for months.
- Pre-Clinical Efficacy: In vivo studies in pancreatic cancer mouse models confirmed that the film-delivered GEM effectively regressed tumor growth, delayed relapse, and showed no observed resistance, outperforming standard systemic delivery in both duration and therapeutic efficiency.
- Biodegradable and Surgery-Friendly: The film is implantable and fully biodegradable, removing the need for surgical retrieval after treatment. It retains enough mechanical strength to be handled and degrades naturally after drug release is complete.
- Reduced Side Effects: By delivering GEM directly to the tumor site, the system significantly reduces systemic exposure, minimizing side effects commonly associated with chemotherapy.
The implant consists of a solvent-cast polymeric film prepared using a blend of chitosan and PVA, with GEM incorporated into the polymer matrix. The drug is mixed with a chitosan solution and combined with PVA under stirring to ensure uniform dispersion. The mixture is then poured into a mold and air-dried to form thin films. A crosslinking step with TPP is performed to enhance mechanical strength and control the release profile.
The resulting film exhibits a gradient in drug concentration profile, with higher retention in the core and reduced concentrations near the two external surfaces of the implant. This structure supports prolonged, sustained release of GEM over a month. The film is flexible, easy to implant, and remains stable under physiological conditions. In vivo testing in pancreatic cancer models showed that the film effectively reduced tumor size, prevented relapse and maintained therapeutic levels of the drug locally, without requiring systemic administration or follow-up procedures for implant removal.
The prototype has been synthesized and validated through in vitro and in vivo experiments in pancreatic cancer mouse models. It has shown superior mechanical performance, controlled drug release and enhanced antitumor activity compared to traditional systemic GEM treatment. The system is stable under physiological conditions and suitable for further clinical evaluation and scaling.
4
This technology presents an important breakthrough in cancer treatment by enabling safe, localized delivery of chemotherapy drugs directly to tumors. It minimizes side effects and improves survival. Its adaptable design also holds promise for tackling other solid tumors, making it highly valuable for public health and cancer care.
- Cancer therapeutics (especially pancreatic cancer)
- Implantable drug delivery systems
- Localized chemotherapy platforms
- Biodegradable medical devices
- Post-surgical relapse prevention tools
Geography of IP
Type of IP
202421000320
562599