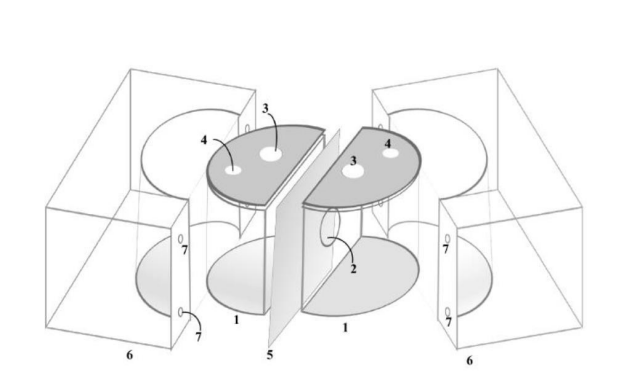
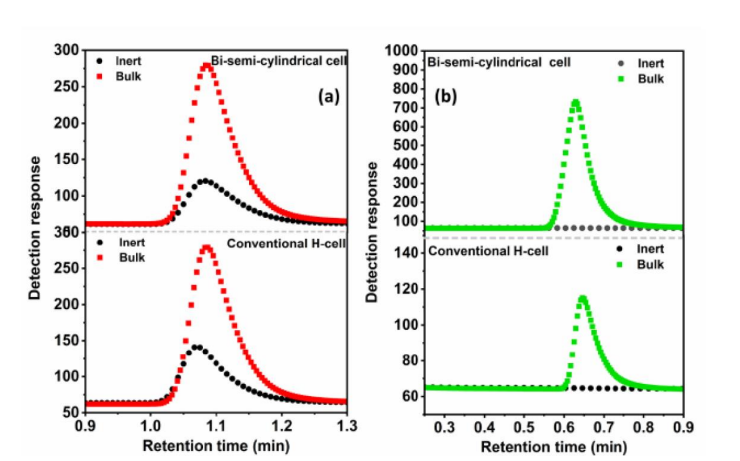
The present invention introduces a bi-semi-cylindrical electrochemical cell tailored for high-efficiency electrocatalytic small molecule activation, such as water electrolysis, CO₂ reduction, and nitrogen fixation. It addresses limitations of traditional H-cells by significantly reducing the ion travel distance between electrodes and incorporating ion-selective membranes for enhanced reaction control. This results in improved charge transfer, reduced gas crossover, and faster electrolysis kinetics.
Conventional H-cells, widely used for electrochemical studies, suffer from large diaphragm distances and non-selective separators like glass frits. These design flaws lead to slower ion transport, reduced reaction kinetics, and mixing of evolved gases, compromising efficiency and selectivity in processes such as water splitting and gas evolution reactions.
- Minimized Ion Migration Path: The flat surfaces of the two semi-cylindrical compartments face each other directly, separated only by a thin membrane, drastically shortening the ion travel path and boosting reaction rates.
- Selective Ion Exchange Capability: It integrates specially chosen polymer membranes (e.g., Nafion®, PVDF) allowing tailored transport of cations or anions, depending on the application. This enhances reaction selectivity and performance.
- Leak-proof and Modular Construction: The two compartments are clamped using durable nylon blocks and tightened with bolts, forming a robust, modular, and reusable system that avoids electrolyte leakage and gas crossover.
- Versatility across Reactions: This setup is suitable not only for water electrolysis but also for electrocatalytic CO₂ and N₂ reduction, demonstrating high adaptability across research and industrial settings.
The fabricated prototype consists of two glass/acrylic semi-cylindrical chambers, each 7 cm in height and 4 cm in radius. These are joined via a central membrane, enclosed using precision-machined nylon blocks. Holes are provided for electrodes and gas outlets. The electrochemical setup includes standard three-electrode configurations with a working electrode (carbon paper), counter electrode (Pt-wire), and reference electrode (Ag/AgCl) in 1M KOH. Performance was assessed using chronoamperometry and gas chromatography.
The technology is prototype-ready and has been validated under laboratory conditions. It is now poised for scale-up, field testing, and potential commercial partnerships.
4
The invention paves the way for more efficient and cost-effective green hydrogen production, essential for decarbonization and energy sustainability. Its modularity and enhanced efficiency make it suitable for scalable applications in energy, environment, and chemical synthesis, as well as for academic research and laboratory teaching.
- Green Hydrogen Production: Enables efficient and scalable hydrogen generation from water electrolysis, supporting the transition to clean energy
- Electrochemical Research and Education: Serves as a robust, modular platform for conducting advanced electrochemical experiments in academic and research laboratories
- Renewable Energy Systems: Integrates into broader renewable energy setups, such as solar-driven electrolysis and energy storage solutions
- Water Splitting and Fuel Cell Technologies: Enhances the study and development of water splitting devices and complements hydrogen fuel cell advancements
- Electrochemical Conversion of CO₂ and N₂: Facilitates the electrochemical reduction of carbon dioxide to fuels/chemicals and nitrogen to ammonia, advancing sustainable chemical manufacturing
Geography of IP
Type of IP
202421024340
563608