The invention relates to a pincer ligand and its palladium complex designed for remote C–H activation of arenes and heterocycles. The system employs non-covalent host–guest coordination to achieve site-selective meta-olefination without covalently attached directing groups. It includes a method for synthesizing the pincer ligand, forming the palladium catalyst, and performing C–H activation under mild conditions with high yield, selectivity, and template recyclability.
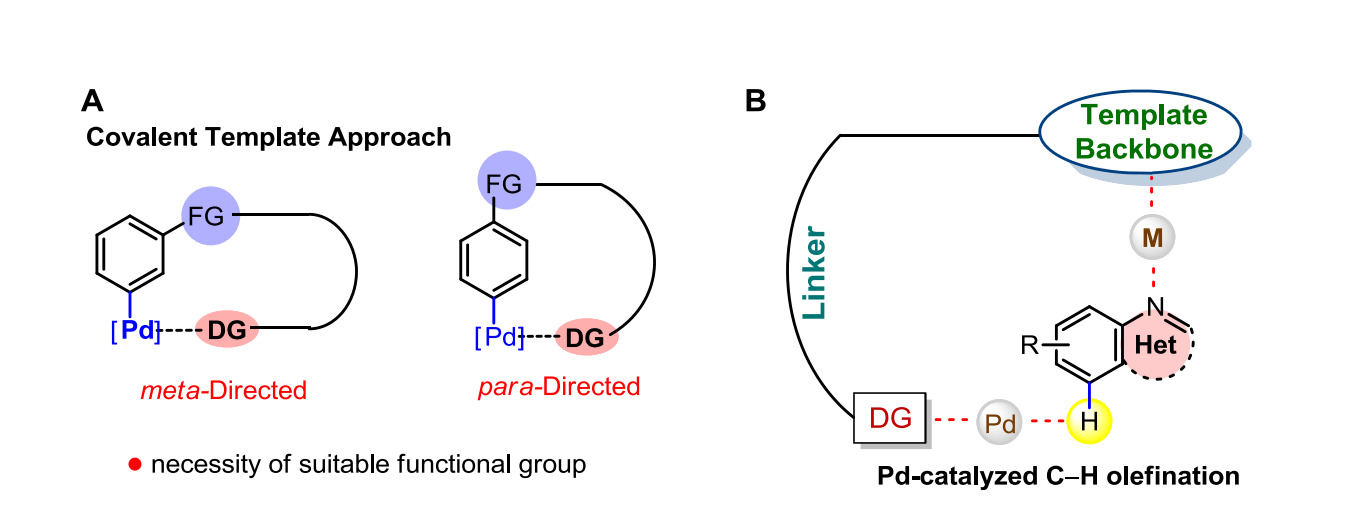
Conventional C–H functionalization strategies rely on covalently attached directing groups (DGs), which require specific functional groups for attachment, involve additional steps for installation and removal, and are often unsuitable for small or electronically sensitive heterocycles. These limitations hinder remote site-selective functionalization and reduce efficiency in complex molecule synthesis. The invention addresses this by developing a pincer ligand system that enables non-covalent, host–guest-type coordination for remote C–H activation, overcoming the drawbacks of covalent DG strategies.
- Design of Smart Ligand System: The invention introduces pincer ligands that enable remote C–H activation through host–guest non-covalent interactions. These ligands eliminate the need for covalently attached directing groups and are effective across varied electronic environments.
- Palladium Catalyst Framework: The catalyst comprises palladium complexed with the pincer ligand, synthesized in one step under mild conditions. This catalyst demonstrates high reactivity and selectivity in meta-C–H olefination reactions.
- Catalyst Preparation and Purification: The palladium catalyst is prepared using standard reagents under mild conditions and purified by column chromatography. The process is simple, reproducible, and suitable for laboratory-scale synthesis.
- Template Recyclability: The ligand templates are recovered after reactions with up to 92% efficiency using mild conditions. These recovered templates retain their structural integrity and catalytic performance for reuse.
- Versatile Substrate Scope: The technology supports high-yield and selective meta-olefination of substrates like quinoline, thiazole, and pyridine. It tolerates various functional groups and is suitable for late-stage functionalization.
The prototype consists of a pincer ligand–palladium catalyst synthesized by reacting the designed pincer ligand with Pd(OAc)₂ in acetonitrile at 60°C for 5 hours. The resulting complex is purified using column chromatography and tested in meta-selective C–H olefination of heterocycles under standard conditions (80°C, 36 h) with N-acetyl glycine and AgOAc as co-reagents in HFIP solvent. The system was successfully demonstrated on substrates such as quinaldine, benzoxazole, and pyridine derivatives, achieving high yields (up to 94%) and excellent regioselectivity (up to 20:1).
The technology has been successfully demonstrated at the laboratory scale with multiple symmetrical and unsymmetrical pincer ligands. Catalytic meta-C–H olefination of various heterocycles has been achieved with high yields (up to 94%) and selectivity (up to 20:1). Template recovery protocols have been validated, and the system has been tested across diverse substrates, confirming its reproducibility and efficiency.
5
The invention offers a sustainable and efficient method for remote C–H functionalization without the need for covalent directing groups. Its applicability to pharmaceutically relevant heterocycles enables streamlined synthesis of complex molecules, reducing waste, cost, and time in drug development and fine chemical manufacturing. The recyclable template further supports green and scalable chemistry practices.
- Pharmaceutical intermediate synthesis
- Fine and specialty chemical manufacturing
- Late-stage functionalization of heterocycles
- Green and sustainable catalysis
- Medicinal and process chemistry
- Ligand and catalyst design research
Geography of IP
Type of IP
201821019668
366142