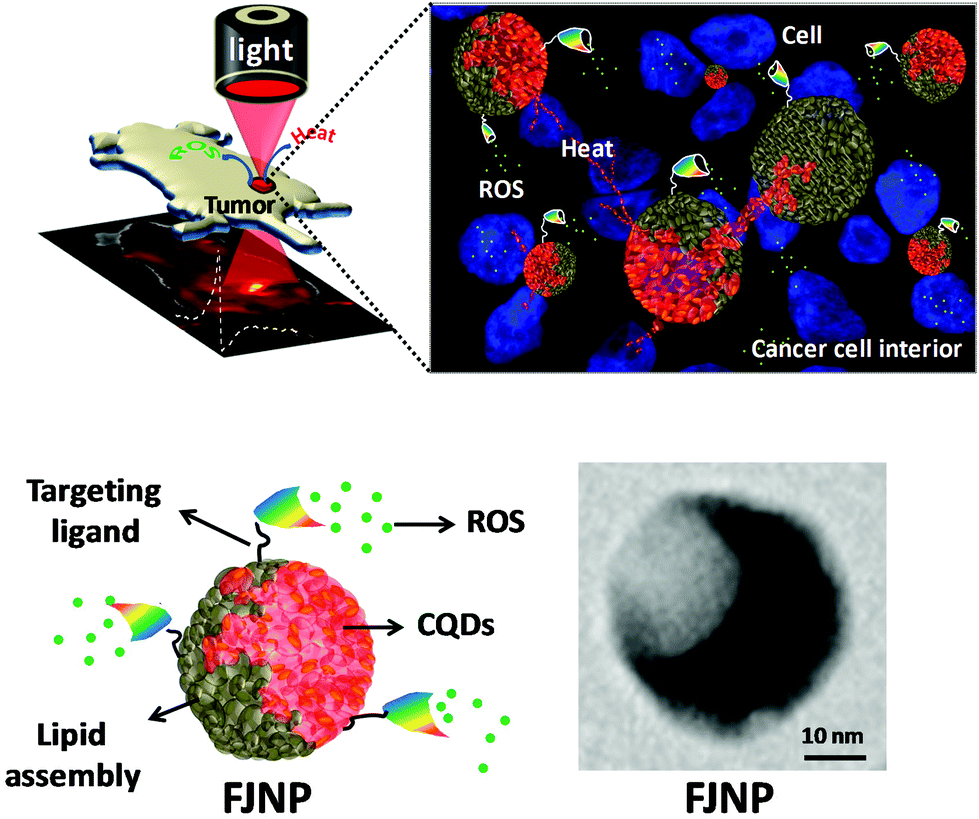
This invention introduces a new biodegradable nanohybrid called FJNPs, made up of red fluorescent carbon dots (C-dots) and liposomal nanopitchers, designed for deep tissue imaging and targeted cancer therapy. These C-dots, attached to liposomes, convert near-infrared (NIR) light into heat, leading to cancer cell death and tumor reduction. Additionally, the nanohybrids can be functionalized with Folic Acid (FA) to enhance targeting of cancer cells. FJNPs and FJNPs-FA improve cellular uptake, generate reactive oxygen species, enable precise tumor detection, and are biocompatible. Using 808 nm light safely, they allow for localized tumor treatment with minimal side effects.
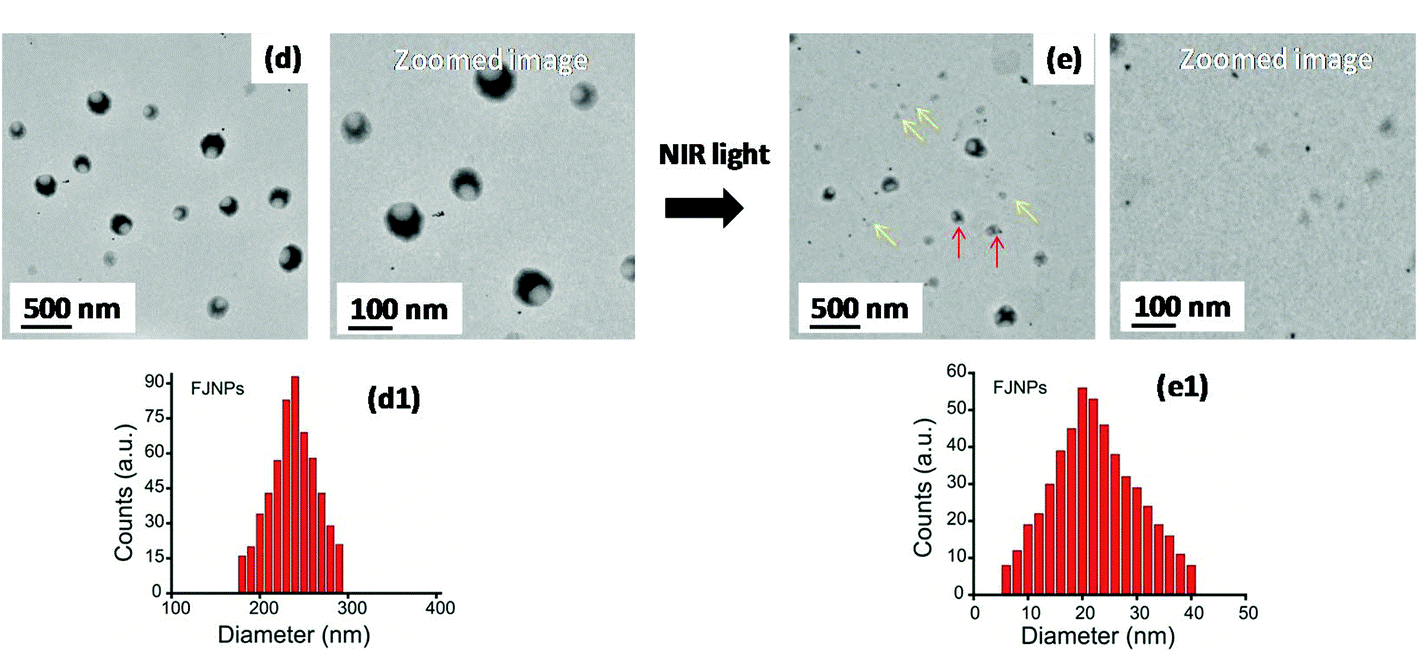
Figure (1) Transmission electron microscopy (TEM) images and particle size distribution (measured by DLS) of synthesized FJNPs (d and d1) before NIR light exposure and those of collapsed FJNPs (e and e1) after 5 minutes of NIR light exposure; yellow arrows show collapsed nanoparticles, whereas red arrows indicate partially decomposed particles; (2) Schematic showing the assembly of liposomal nanopitchers, namely FJNP, and its usages in photo-driven tumor diagnosis and tumor growth inhibition through localized photothermal heat (red colour) and Reactive Oxygen Species (ROS, green colour) in the tumor environment under NIR light irradiation.
The main challenge in developing effective theranostic systems for cancer is achieving selective targeting and localized therapy without harming healthy tissues. Existing nanosized formulations, including inorganic nanostructures and organic dyes, show promise but face issues like photobleaching, aggregation, slow degradation, low uptake, and poor image resolution. UV light causes phototoxicity, while prolonged NIR exposure can lead to tissue injury and requires high power density for effectiveness. Current photothermal therapies focus mainly on heat generation, overlooking the role of Reactive Oxygen Species (ROS) in cell death.
Thus, there is a need for a system that integrates diagnostics and therapy safely and effectively, utilizing non-metallic nanoparticles, which have not been explored before.
- Enhanced Photothermal and ROS Response: The C-dots efficiently convert NIR light into heat, achieving significant photothermal response within five minutes of exposure. This process also generates ROS, which aid in cancer cell death through membrane rupture and oxidative stress.
- Targeted and High Cellular Uptake: This approach achieves targeted and high cellular uptake due to functionalization with Folic Acid (FA), enhancing therapeutic efficacy.
- Biodegradable and Biocompatible: Due to a non-metallic, biodegradable core made from lipids and cholesterol, it is biocompatible.
- Cost-effective: Simple and scalable synthesis, with the one-pot synthesis method, simplifies the production of FJNPs, making it cost-effective.
- Deep Tissue Imaging and Therapeutic Efficacy: Facilitated by the red fluorescent emission of C-dots, it improves diagnostic capabilities.
NA
In vivo studies of red fluorescent C-dots liposomal nanopitchers (FJNPs) in mice demonstrate significant tumor reduction (from 250 mm to 18 mm) and high cancer cell death rates (90-97%) under NIR light exposure.
The FJNPs show high biocompatibility, with cell viability assays indicating viability rates of about 85-95%.
- Enhanced Cancer Treatment: Engineering liposomal nanotheranostics offer a non-invasive and localized treatment option for cancer, potentially minimizing damage to healthy tissues. This could improve patient outcomes and quality of life.
- Improved Diagnostic Accuracy: The ability of engineering liposomal nanotheranostics to provide precise tumour imaging enhances diagnostic accuracy. This can lead to earlier detection of cancers and better monitoring of treatment progress.
- Reduced Healthcare Costs: By offering targeted therapy and efficient treatment modalities, FJNPs have the potential to reduce overall healthcare costs associated with cancer treatment. This includes fewer hospitalizations, shorter recovery times, and potentially lower long-term care needs for patients.
- Cancer Therapy: This approach offers targeted photothermal therapy for localized tumour ablation and regression.
- Enhanced Biomedical Imaging: It facilitates accurate tumour diagnosis and monitoring with enhanced biomedical imaging.
- Efficient Drug Delivery: Therapeutic agents targeted delivery, with minimized side effects, enhances drug delivery efficiency.
- Biocompatible Nanomaterials: Development of biocompatible materials for various biomedical applications.
- Theranostics: It integrates diagnostic and therapeutic capabilities in a single platform for personalized medicine.
Geography of IP
Type of IP
202121022522
486170