Point Of Care Sickle Cell Test
This invention is a microscopy-based test designed to diagnose sickle cell disease (SCD) and distinguish between normal, sickle cell trait, and sickle cell disease (SCD) samples. It involves extracting and analyzing the morphological features of Red Blood cells in a hypoxic environment to classify blood samples as usual, sickle cell trait, or sickle cell disease. Additionally, the invention offers formulations, methods, kits, and devices.. It offers advantages such as distinguishing between disease and trait samples with high sensitivity and specificity, providing automated image processing for diagnosis, being more affordable than existing confirmatory tests (HPLC or molecular test), and delivering rapid results (less than an hour) with a single microscopy-based test. The test can work with a finger prick of blood, making it more accessible than traditional methods requiring larger blood samples.
Sickle cell disease (SCD) is a debilitating genetic disorder impacting millions globally, predominantly among individuals of African, South, or Central American (especially Panamanian), Caribbean, Mediterranean (including Turkish, Greek, and Italian), Indian, and Saudi Arabian descent. In Sub-Saharan Africa, approximately one-third of the indigenous population carries the Hb-S gene, a hemoglobin gene mutation responsible for the sickle-shaped red blood cells characteristic of SCD. In India, SCD has a profound impact on tribal communities, with 50,000 out of the 300,000 children born with SCD annually worldwide being Indian. Tragically, 20% of these tribal children succumb to the disease before their second birthday, 30% by age 25, and 50% by age 40. Similarly, data from the CDC indicate that sickle cell anemia affects about 1 in every 500 African Americans, while the sickle cell trait is present in about 1 in every 12. The average life expectancy for individuals with SCD is 30 years below the norm, underscoring the critical need for early and accurate diagnosis to prevent complications such as severe infections and to facilitate life-saving treatments. However, current two-stage diagnostic methods—hemoglobin electrophoresis, genetic testing, and high-performance liquid chromatography (HPLC) being the confirmatory tests—are time-consuming, complex, and require specialized laboratory settings operated by highly trained healthcare professionals. Effective treatment of SCD and its complications often necessitates rapid diagnosis, particularly in settings outside conventional medical facilities. Therefore, a point-of-care diagnostic method that can be utilized by laypersons (i.e., individuals with minimal or no formal medical training) is crucial. Such a solution would enable prompt treatment decisions, significantly improving patient outcomes and reducing the healthcare burden in resource-limited settings.
- High Accuracy: The system achieves remarkably high concordance (100%) with HPLC or distinguishing between healthy (HbA) and sickle cell disease (HbS) samples, as well as between sickle cell trait and sickle cell disease samples.
- Automation and Technician Independence: By leveraging image processing, this technology eliminates the need for technicians to analyze manually, streamlines the diagnostic process, and reduces the potential for human error.
- Enhanced Affordability: This solution is projected to be considerably more cost-effective than established confirmatory tests like hemoglobin electrophoresis and high-performance liquid chromatography (HPLC).
- Rapid Single-Step Testing: This microscopy-based approach delivers results in a single test, surpassing the current multi-step process typically involving a solubility-based screening test followed by confirmatory HPLC or electrophoresis. 5. Minimal Blood Sample Requirement: The assay functions with a finger prick's worth of blood, unlike traditional methods that often necessitate larger venous blood draws.
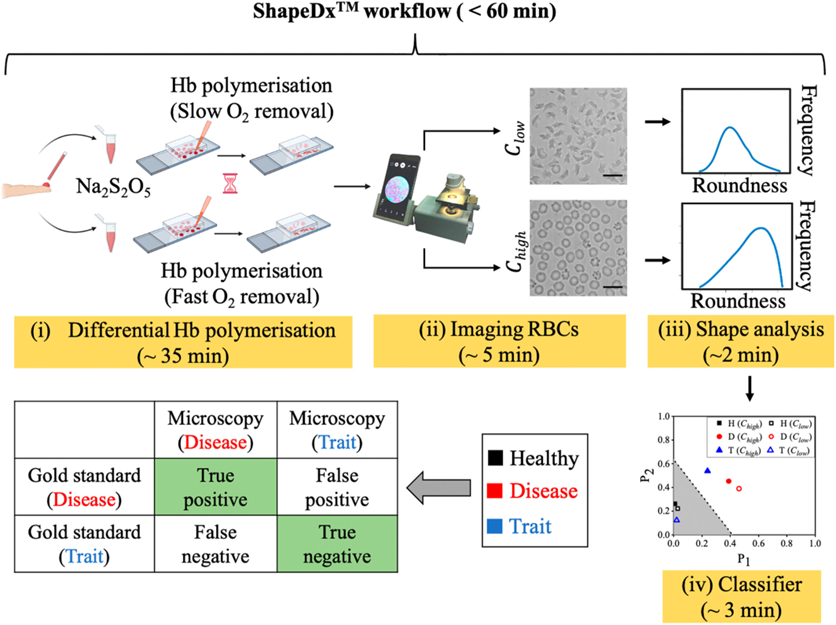
- ImagingChamber Design: The technology employs an imaging chamber with precisely defined dimensions, facilitating controlled and efficient movement of red blood cells during sickling. The chamber's height is maintained at ~30 μm, ensuring accurate imaging and analysis of the sickled cells.
- Innovative Sickling Formulation: The invention utilizes two specific concentrations of a chemical oxygen scavenger, such as sodium metabisulphite (0.1%-0.5%), within a diluting agent to create a hypoxic environment. This triggers the sickling process in red blood cells, allowing for controlled and reproducible results.
- Automated Image-Based Analysis and Classification System: Incorporating a mobile phone microscope and ImageJ software, the technology extracts detailed morphological parameters of red blood cells, such as area, roundness, and solidity. An automated system then uses these parameters to differentiate normal from HbS samples and further classify HbS samples into sickle cell trait and disease categories.
- Point of Care Application: Offering a robust point-of-care solution, the technology facilitates immediate diagnosis, reducing the need for multiple hospital visits. This feature is invaluable for field research, community health screenings, and rapid diagnostic interventions, providing timely and effective healthcare delivery.
The testing process is designed to be straightforward (Figure 1). First, a small blood sample is added to two specific concentrations of the sickling formulation and incubated for a predetermined time. We capture images of these two blood samples after a pre-determined time and compare the RBC shapes in these two images to arrive at a diagnosis. We use a custom-designed mobile phone microscope to capture images of the RBCs before and after exposure to the sickling formulation. These images are then analyzed using an open-source software called ImageJ, which makes them accessible. By analyzing morphological parameters, the system can differentiate between normal and SCD (HbS) samples and even further classify HbS cases into SCT or disease.
- Population-level Screening: This invention could enable large-scale screening programs to identify potential Sickle Cell Disease (SCD) patients, particularly beneficial in areas lacking access to traditional diagnostic tools.
- Point-of-care Diagnosis: The portability of the device allows for diagnosis at the point of care, eliminating the need for patients to travel to hospitals with specialized equipment (like HPLC machines) often unavailable in remote areas. This can significantly improve access to SCD diagnosis, especially in resource-limited settings.
- Reduced Burden on Hospitals: By enabling more decentralized diagnosis, this invention could alleviate the strain on hospital resources like HPLC systems, allowing them to focus on more complex cases.