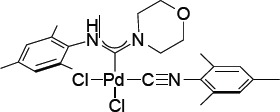
This invention relates to the synthesis and application of novel palladium acyclic diaminocarbene (ADC) complexes of the general formula cis-[(R¹NH)(R²)methylidene]PdCl₂(CNR¹), where R¹ = 2,4,6-trimethylphenyl and R² = NC₅H₁₀ (2), NC₄H₈ (3), or NC₄H₈O (4). These complexes are investigated as efficient Precatalysts for Hiyama coupling reactions, specifically for Csp²Csp cross-coupling between aryl iodides and triethoxysilylalkynes. Additionally, the invention demonstrates their use in a tandem one-pot, fluoride-free Hiyama coupling/cyclization reaction to synthesize biologically relevant benzofuran derivatives from 2-iodophenol and triethoxysilylalkynes. This catalytic system offers a streamlined and efficient approach to constructing complex organic molecules without the need for external fluoride activators.
There remains a need for greener and more versatile catalytic systems in cross-coupling chemistry. The Hiyama coupling, using low-toxicity organosilicon reagents, offers a greener alternative to Suzuki and Stille reactions but remains unexplored with transition metal complexes bearing acyclic diaminocarbene (ADC) ligands. No reports exist on their use in one-pot Hiyama coupling/cyclization to synthesize benzofuran derivatives. Unlike N-heterocyclic carbenes (NHCs), ADC ligands are more accessible, flexible, and may offer unique catalytic reactivity. This underscores the potential of transition metal-ADC complexes in developing efficient, green routes to benzofuran frameworks.
- Innovative Catalyst System: The invention employs palladium-based acyclic diaminocarbene (ADC) complexes as highly effective catalysts for Hiyama cross-coupling reactions, offering enhanced stability and reactivity compared to conventional ligands.
- One-Pot Tandem Reaction Approach: The method integrates Csp²-Csp Hiyama coupling with in-situ intramolecular cyclization in a single reaction vessel, minimizing purification steps and streamlining synthesis.
- Sustainable Reaction Medium: Reactions are carried out in a mixed dioxane– water solvent system using non-toxic organosilanes, aligning with green chemistry principles and reducing environmental footprint.
- High Functional Group Tolerance: The process is compatible with a wide variety of functional groups (e.g., halogens, electron-donating and electron-withdrawing substituents), enabling structural diversity in the products.
- Access to Biologically Relevant Scaffolds: The methodology facilitates the synthesis of benzofurans, which are important heterocycles found in a range of pharmaceuticals, agrochemicals, and bioactive natural products.
- Improved Yield and Atom Economy: The optimized conditions offer moderate to high yields with excellent atom economy, making the process suitable for scale-up and industrial translation.
- Low Catalyst Loading and Cost Efficiency: The catalyst operates effectively at low concentrations, reducing the overall cost of the reaction and minimizing palladium contamination in the final product.
- Versatility across Multiple Domains: The technology is applicable in pharmaceutical R&D, material sciences, and catalysis development-demonstrating broad utility beyond traditional synthetic organic chemistry.
This invention demonstrates a novel, efficient method for synthesizing 2-substituted benzofurans using a palladium-based acyclic diaminocarbene (ADC) complex as a catalyst. The process involves a one-pot tandem Hiyama alkynylation-cyclization reaction between iodophenols and triethoxysilylalkynes in a dioxane-water medium under mild conditions. It enables the selective formation of valuable benzofuran scaffolds with broad substrate scope, good functional group tolerance, and moderate to high yields-highlighting its potential for industrial application in the synthesis of bioactive and functional aromatic compounds.
This invention is about a new type of reaction that would give convenient access to a variety of fine chemicals relevant to chemical, agro and pharma industries. The patented methodology uses cheap and environmentally friendly organosilicon compounds whose applications are in vogue these days.
4
The developed methodology for synthesizing benzofuran derivatives holds significant societal impact by enabling efficient, eco-friendly production of bioactive molecules with pharmaceutical potential, including anticancer, antimicrobial, and CNS- active agents. Using a sustainable, water-compatible system and non-toxic organosilanes, it aligns with green chemistry principles. The cost-effective catalyst and simplified conditions make the technology accessible for wider adoption in industry and academia, accelerating innovation in health, agriculture, and materials science.
- Pharmaceutical industry
- Agrochemicals
- Materials science
- Fine chemicals
- Catalysis research
Geography of IP
Type of IP
16/168,434
11033889