A method of isolating an antibody is disclosed. The method comprises contacting a hydrophohic chelator, a non-ionic detergent and metal ion so as to generate an aggregate comprising the hydrophobic chelator, the detergent and the metal ion, and contacting the aggregate with a medium comprising the antibody under conditions that allow partitioning of the antibody into the aggregate. Kits for isolating the antibody are also disclosed.
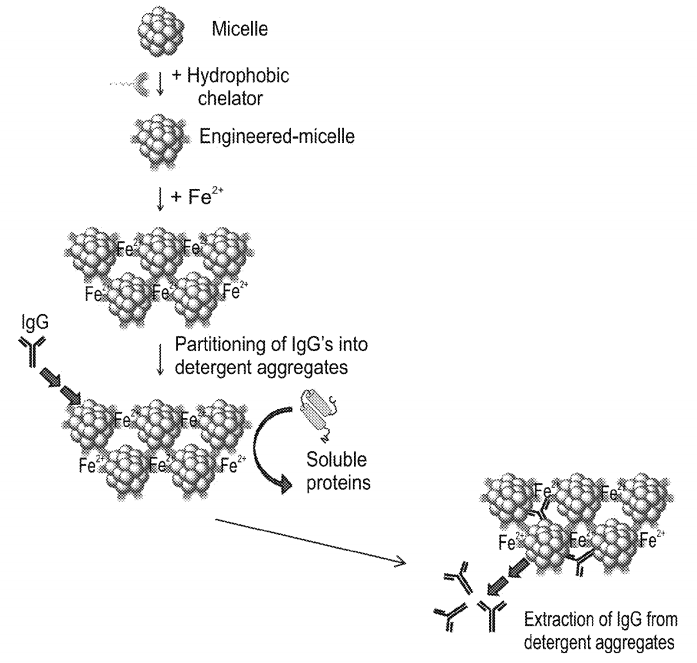
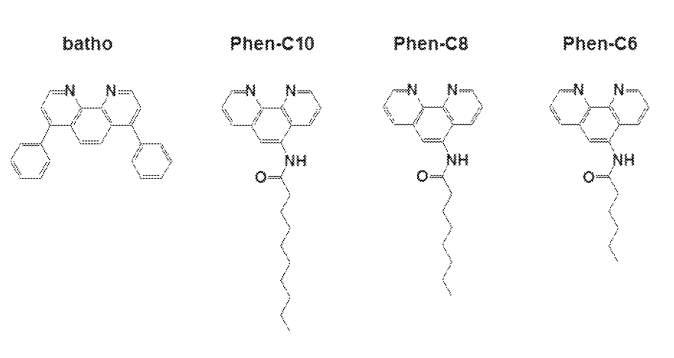
A new concept for antibody purification was uncovered. Human immunoglobulin G (hIgG) and mouse IgG partition almost quantitatively (~95% by densitometry) into aggregates of non-ionic detergents, metal ions and hydrophobic chelators whereas the majority (>85% by densitometry) of non-lgG proteins (ie. impurities) are rejected. The process is highly specific as it relies on the presence of the chelator and the metal. Antibodies that are adsorbed or embedded within the aggregates can be extracted without concomitant dissolution of the aggregates and lead to purer IgG preparations (~95% by densitometry). The overall yield of the process that includes: IgG partitioning and extraction range between ~40-46% (by densitometry).
- Non-chromatographic method, reduction in raw material cost, high purification yield, short purification time, suitable for large scale purification.
- A kit for precipitating antibody comprising a hydrophobic chelator and a metal ion.
It is a kit containing a hydrophobic chelator and a divalent metal ion for the purification of antibodies.
3
It is a non-chromatographic method for the purification and structural determination of antibodies. It would be highly efficient and cost effective.
Structural biology, biochemistry, biotechnology, drug delivery.
Geography of IP
Type of IP
16/611,859
US 12,091,445 B2