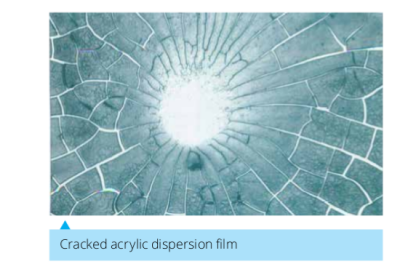
We all want our painted walls to have uniform colour and be free of surface cracks or warps. This is achieved by formulating paints that dry homogeneously and do not crack or de-bond from the surface during drying. Paints typically contain a solvent that evaporates during drying, a polymer binder that forms a continuous film at the end of the drying process, pigments that give colour and a number of additives to improve the toughness or impart special properties to the film.Traditional paints and coatings use organic solvents but recent concerns of their impact on human health and environment has forced the industry to move towards water-based products. However, the organic solvents play a critical role of softening the binder, which enable them to form a homogenous film. Water-based paints, on the other hand, can suffer from problems of cracking or surface chipping. Our work focuses on the understanding of the drying, film formation and cracking mechanisms in paints and coatings with a goal to relate the physics of the processes to the final film properties. Using a judicious mix of theory and experiments, our group showed that cracking in
paints is caused by a balance between the elastic energy stored in a stressed drying film and the surface energy created during cracking. The theory was able to explain the cracking behaviour in diverse systems, extending from paints containing soft particles to very hard ceramic systems containing metal oxide particles. Our group extended the theory to address the cracking behaviour in a mixture of hard and soft particles, which are closer in composition to the commercial paint. We also investigated the role of flaws, such as holes, in the drying film and its influence on the cracking behaviour. More recently, we have been investigating the dynamics of crack propagation. Our work continues to be supported financially by the Department of Science and Technology, Government of India.
Prof. M S Tirumkudulu
