Increasing level of CO 2 in the environment owing to anthropogenic activities is one of the pressing global issues that needs immediate resolution. Ever increasing development of industries and human population will produce more and more CO 2 emission by continuous consumption of fossil fuels. The immediate challenge is to improve the current technologies which can capture CO 2 efficiently and convert it into useful sustainable fuels (i.e. methane and methanol) in presence of natural resources such as water and sun light. For effective carbon dioxide capture and sequestration (CCS) the reusability of the capture material is an essential conditions. The current technologies for CCS using aqueous monoethanolamine solutions chemically react with CO2 and do not expedite regeneration of the materials.In search of renewable materials, covalent organic frameworks (COFs) were introduced in to literature in 2005. The COFs are purely organic materials with low mass density, high surface area, high thermal stability and long- range ordered architectures. COFs are rationally designed material consists of π-conjugated 2D sheets formed by strong covalent linkages. The sheets are forming ordered columnar π-arrays and aligned 1D nanochannels by π–π interactions. The arrangement of 1D channels within COFs
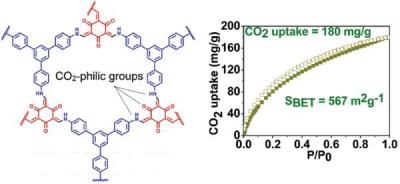
provide an opportunity to modify the pore size and introduce CO 2 -philic functional groups such as amine, aminal, azo and imine etc., for high and selective adsorption of CO 2 . We have successfully synthesised and characterised imine and β-ketoenamine based porous and fluorescent covalent-organic frameworks. These COFs show high thermal stability (~ 450°C) and high surface area of upto 756 m 2 g −1 (BET) and 1515 m 2 g −1 (Langmuir). To understand the ease of getting high surface area,
the COFs are decorated with bulky isopropyl (iPr) group and found that iPr group on the aromatic ring reduces the network entanglement and hence,
increases the surface area. More importantly, this frameworks exhibit a very high carbon dioxide uptake capacity of 180 mg g −1 (18 wt %) which is the third highest uptake in the family of imine based COFs. Besides, it has been demonstrated that the same materials can also be employed for the explosive nitroaromatic compounds detect. Our group is currently addressing the modification and introduction of CO 2 -philic functional groups on COFs to enhance the CO 2 uptake and subsequent conversion into methanol.
Prof. R Murugavel
