Human malaria is caused by parasites (Plasmodium falciparum and Plasmodium vivax) that are introduced into the body by the bite of a female Anopheles mosquito. These parasites first invade the liver and then the red blood cells (Fig. 1). In both the liver and red blood cells, parasites multiply so rapidly that one infected person can have as many as several billion parasites in his/her blood cells. To carry out this massive multiplication, parasites need to copy themselves. This is done by synthesising large quantities of the biological molecules that they are made of: DNA, RNA, proteins, carbohydrates and lipids.Our lab studies the biosynthesis of one of the biomolecules mentioned above:proteins. Protein synthesis is carried out by a complex machinery called the ribosome, using messenger RNA (mRNA) molecules as information carriers. Ribosomes of the malaria parasite recognise mRNA by binding a structure called the ‘cap’ on one end of the linear mRNA molecule and then they scan the linear mRNA until they reach a sequence called the start codon. At the start codon, ribosomes begin to synthesise proteins, and finally terminate at
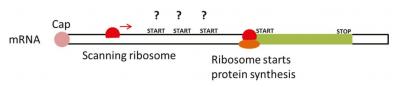
stop codons. Malaria parasites are not alone in carrying out protein synthesis in this manner; indeed, all eukaryotes do the same.One big difference between other eukaryotes and one of the malaria parasites P. falciparum is the fact that while other eukaryotes have a single start codon (maximally two start codons) in their mRNAs, our lab has shown that P. falciparum parasites have an average of 5 start codons in their mRNAs. Because of this, the parasite ribosome machinery has many start codons to choose from and could begin protein synthesis at many different places. If protein synthesis does not begin at the correct start codon, the information to make the protein would be wrong and the protein would be synthesised incorrectly. It is therefore essential to choose the correct start codon and P. falciparum is faced with a problem of choosing the correct one from the many decoys. Fig. 2 shows this as a schematic. Our lab showed that, similar to other eukaryotes, the sequences surrounding the start codons tell the ribosomes which are the correct start codons. We also showed that in some cases, the ‘extra’ start codons can in fact prevent the ribosome from reaching the correct start codon. This leads to an important question. During multiplication in the red blood cells, parasite proteins are made in large amounts. If the parasite ribosome is prevented from reaching the correct start codon, how are these parasite proteins synthesised? Initial results suggest that the parasite ribosome can skip over the extra start codons to reach the correct start codon by two processes called leaky scanning and re-initiation. How does this work help towards controlling the disease, malaria? One strategy could be to understand the key differences between protein synthesis in the parasite and in the human host. We already know that the parasite ribosome must handle extra start codons by skipping them, while the human ribosome does not need to do this. We hypothesise that parasite-specific factors may do this and are in the process of identifying and inactivating these factors. If we can inactivate these factors with drug-like compounds, it might be possible to kill malaria parasites and take the first steps towards potential new drugs against this deadly disease.
Prof. S Patankar
