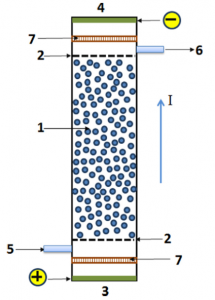
Disinfection of drinking water, swimming pool water, and water used in washing food processing equipment, surgical instrument, food packaging, etc., is an important step from the health consideration. Variety of disinfectants such as chlorine, sodium hypochlorite and bleaching powder are used for this purpose. These generate free chlorine, which is a very effective biocide even at very low concentrations. However, when concentration of chlorine in water is high, it reacts with humic acids present, producing polychloro-derivatives which are potent carcinogens. Hence, it is necessary to limit concentration of chlorine in water to a few parts per million.By performing electrolysis of very low concentrations of sodium chloride in a continuous flow reactor, it is possible to regulate the concentration of chlorine in water well below its harmful limit. Electrolysis is therefore a preferred method for performing disinfection. However, low conductively of water gives rise to large ohmic losses and consequently high power consumption per unit volume of the disinfected water. Open bipolar electrolysis is a technique which can overcome this limitation. A typical bipolar reactor is as shown in the figure. Here, the metal electrode particles are suspended in a fluidised bed. Electric current is fed from one end and flows through the bed. The ohmic drop produced by the current polarises the electrode in such a way that one side of each electrode acts as cathode and the opposite side as anode. A large surface area of electrode is made available in form of a large number of small particles. Moreover, since a large amount of current passes through the electrode body which has high conductivity, the effective conductivity of the system is much higher and power consumption is considerably reduced. It is therefore very economical technique for water disinfection.Our work involves studying bipolar electrolysis of water containing low concentrations of chloride ions with a purpose to understand the mechanism of electrolysis and also find the ways to improve the rate of electrolysis and reduce the power consumption. Along with standard techniques such as
voltammetry and chronoamperometry on rotating disc electrode, we work on different designs of bipolar cells. These studies will be useful in design of large scale and efficient bipolar reactors for water disinfection.
Prof. V A Juvekar
