In a mammalian system, not all the cells are continuously growing and dividing but many of them remain in a quiescent state even though they reside in a rich growth factor medium. Similarly, in a malignant tumor the population of cell had been found to be extremely heterogeneous. Usually the tumor contains cells ranging from highly proliferative to quiescent category, and thus these cells respond quite differently under chemotherapy treatment. Most often the aggressive cells die, but the quiescent cells somehow survive the chemotherapy. Thus, cancer relapses as soon as the chemotherapy is withdrawn.
In this context, recently it has been reported that quiescent leukemic cells (QLCs) showed proliferative response once they were treated with inhibitor of p38 protein, and consequently the response to the chemotherapy was far better. This result distinctly demonstrates that signaling pathways within a mammalian cell such as p38 signaling pathway controls the transition from quiescent state to the proliferative state by significantly manipulating the cell cycle of the mammalian cells. The protein p38 is quite well studied in the literature, and found to be influential in controlling proteins related to different phases of the cell cycle. Thus, the regulation of cell cycle events by p38 signaling pathway is extremely complex, and a better understanding of it is necessary by employing improved quantitative tools.
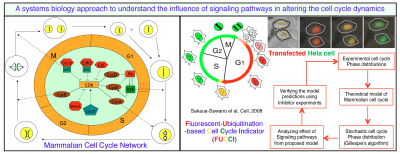
Keeping this in mind we intend to study how the p38 signaling pathway or any other related signaling pathway that controls the level of p38 protein alters the cell cycle in mammalian cells in a cell type specific manner. For specific mammalian cell types where, the cell cycle control system is functioning reasonably well, we will first try to observe how the duration of different phases of the cell cycle are changing in absence or presence of p38 inhibitor at the single cell level by inserting FUCCI (Fluorescence Ubiquitin Cell Cycle Indicator) reporter. This experiment will provide us with the information of the distribution of time spent in the different phases of the cell cycle (i.e., G1 and S-G2-M phase) as well as the distribution of the total cell cycle period.
On the other hand, we are currently building a mathematical model of the cell cycle regulation based on mass action kinetics while incorporating the required nonlinearity by using the idea of multi-site phosphorylation commonly found in cell cycle network. We will systematically include the connections of p38 signaling pathway to the cell cycle regulation model. We are constructing a data-driven mathematical model of the cell cycle regulation under the influence of p38 signaling pathway. The deterministic mathematical model will be constructed in such a manner that it can be readily convertible to a stochastic framework to reproduce the theoretical distributions of the cell cycle period and phases observed in our single cell experiments. The predictions made at different levels of the mathematical modeling will be verified experimentally. This kind of systems biology approach to resolve such a complex problem will ultimately lead us to some kind of testable hypotheses. Knowledge gained from this study can be tested on some other cell types to see whether the features that we are observing in our selected cellular systems are universal or not. We believe that such system level understanding will be significant to employ novel therapeutic strategies for treating cancer patients in future.
Prof. Sandip Kar
