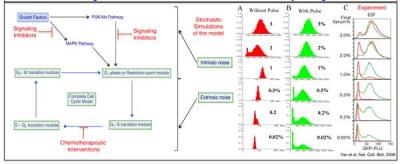
Cell cycle in eukaryotes is a sequence of molecular events that ultimately leads to precise duplication of cells. Most cells of a multi-cellular eukaryotic organism proliferate only when they sense that they are stimulated by specific growth factors above a certain threshold. In cancerous cells, this growth factor sensing mechanism gets perturbed for more than one reason and the cells start to proliferate even when the appropriate signal is absent. Signaling pathways such as PI3K/Akt and MAPK pathways have become a common therapeutic target nowadays in cancer treatment because growth factor mediated signaling is transmitted to the cell cycle machinery via these signaling pathways at different stages of the cell cycle.The process of cell cycle progression under the influence of different signaling pathways is bound to be quite noisy because of intrinsic (for example, caused by low numbers of mRNA and protein molecules) and extrinsic fluctuations (for example, any protein concentration within an individual cell in an asynchronous population of cells, has been found to be extremely heterogeneous). Keeping this in mind, by using theoretical and computational approaches, we are systematically investigating and quantifying the roles of intrinsic and extrinsic fluctuations in the context of cell cycle regulation of the mammalian cells under the influence of different signaling pathways (PI3K/AKT, MAPK) individually and collectively.Cell cycle regulation for the mammalian cells is regulated by a number positive, negative and feed-forward feedback loop motifs. For budding yeast (the simplest eukaryotic organism) cell cycle, experimentally it had been observed that among all the phases, the G1 phase of the cell cycle wasthe noisiest phase. Even for mammalian cells, which are much bigger in size than the budding yeast, it had been reported that the G1 phase is the only phase that depends on cell size. This indicates that the effect of molecular noise and the extrinsic
fluctuation due to varying protein concentrations in a population can be observed to a greater extent during the G1 phase for the mammalian cell cycle as well. The effect of growth factor activated signaling pathways (and the related extrinsic and intrinsic noise) will be mostly effective during the G1 phase progression. We will explore and investigate these ideas by making suitable growth factor induced cell cycle model of mammalian cell and try to quantify the effect of different kinds of noise in the concerned system. We believe that a system level knowledge of the noise regulation in the cell cycle process will definitely strengthen the fundamental understanding of the mammalian cell cycle control machinery that will pave the way for novel therapeutic avenues.
Prof. Sandip Kar
