The interest of the automotive and aviation community in biofuels has surged in the past decade primarily due to a shift in global awareness towards cutting greenhouse gas emissions. This has translated into stringent policies for fuel usage and emission norms. In this context, the research community has shifted its focus towards biofuels as a part of the larger renewable plan for the planet. Integrating the usage of these biofuels to the existing ecosystem of different combustion systems requires an in-depth understanding and precise knowledge about the combustion properties of these fuels at different conditions of elevated temperature and pressure.
Laminar burning velocity is a parameter which is often used to characterise
the reactivity of the fuel, its heat release and flame propagation behaviour.
This data can also be used in designing of fuel delivery systems from a
safety point of view. The validity of proposed fuel chemistry developed by
chemists can also be assessed using laminar burning velocity data. These
fuel chemistry models of high accuracy can be used for accurate modeling
of flames in simulations which helps in predicting pollutant and acoustic
emissions, thus helping in optimisation of combustor designs. Therefore,
this parameter is of vital importance while studying and developing newer
alternate fuels.
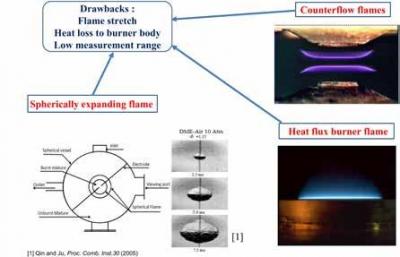
The measurement conditions for this parameter call for planar stretch
free flames without any heat loss. There are various methods available in
literature (counter-flow burner, heat flux burner and spherically expanding
flames) to measure this parameter albeit with some major shortcomings.
Flame stretch, curvature, flame cooling and limited range of measurements
(<450 K for heat flux and counter flow flames) leads to inaccuracies and
scatter of up to 40% in such reported data.
Our group has addressed these shortcomings by developing a new
methodology using meso-scale quartz channels within an uncertainty
band of ± 5%. The flame dynamics inside such channels is studied. Planar
stretch free flames are stabilised at temperatures as high as 650 K (engine
relevant conditions) and burning velocity data is extracted using simple mass
conservation principles.
We have generated accurate data for C1-C5 straight chain alcohols (methanol,
ethanol, n-propanol, n-butanol and n-pentanol) and compared it with
predictions from mechanisms proposed by various research groups globally.
The data is good agreement with the predictions except in the fuel rich
regimes where the over predictions by the mechanisms are quite significant.
Thus, we have concluded that the existing mechanisms need significant
improvement and more accurate modeling of various kinetic parameters.
Prof. Sudarshan Kumar
