Majority of the portable electronic devices used in our day-to-day life, starting from basic cell phone, smart phone, laptop, digital camera, camcorder, tablet, power tools etc. use Li-ion batteries as the power source. Imagine the next generation automobiles also running on such batteries, instead of burning fossil fuels and causing environmental pollution. Yes, there is a definite possibility for the same, leading to saving our environment and having us breathe fresh air, even in the cities. Electric vehicles, as opposed to gasoline vehicles, will also address the scare concerning depletion of sources for fossil fuels and rising prices for the same. In a broader perspective, this is where our research on electrochemical energy storage at the High Temperature and Energy Materials Laboratory, IIT Bombay is primarily focused on.However, the present generation Li-ion batteries are yet not capable of running electric vehicles to the best of the necessities and customer satisfaction. The prohibiting aspects include charging durations (nobody would like to wait for an hour to recharge car batteries during a drive), the
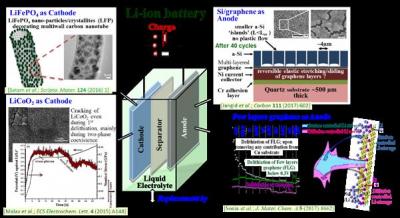
safety concerns (a car cannot have a battery which might just ‘explode’ sometime!), the ‘mileage’ upon full charge and the cost (especially considering that the present generation batteries don’t usually last for more than a couple of years). In more technological terminologies, the present day Li-ion batteries lack in terms of power density, energy density, cyclic stability and safety aspects; as needed for application in electric vehicles. Looking more closely inside a single cell, addressing all these aspects warrant significant contributions from materials science/engineering and materials development. Accordingly, our quest has been towards developing nanostructured electrode materials, such as those based on well-ordered graphenic carbon, active nanoparticles (such as LiFePO 4 , LiNiMnCoO 2 , SiO 2 , Si, etc.) ‘decorating’conducting reinforcements (such as carbon nanotubes or graphene), Si nanowires (having innovative core/shell crystalline/amorphous morphology), etc., by careful optimisation/control of the processing conditions and structural aspects, in order to come up with novel/improved electrodes having considerably greater Li-storage capacities (leading to improved energy density), rate capabilities (i.e., ability to get charged and deliver energy very
fast), cyclic stability (or cycle life) and safety aspects. In addition to the detailed materials and electrochemical studies/engineering, the research also involves in-depth investigations/understandings via use of various innovative in situ techniques (such as stress monitoring during discharge/charge, phase evolutions in electrodes during electrochemical Li-insertion/removal). ‘Full cells’ are being developed based on the results of such investigations, understandings and engineering/development of electrode materials; leading to considerably improved performances on all the aforementioned aspects. Considering that solid-state Li-ion batteries have the potential to be one of the ‘safest’ energy storage systems (but presently have various issues at scientific/engineering levels), more recently focus has also been directed towards the understanding/development of Li-ion conducting solid-state
electrolytes. Looking further ahead and considering possible depletion of Li-based resources in the future, focus has also been directed towards understanding and development of electrode materials for the upcoming Na-ion battery technology (a research and development area, which may be very important in the Indian contexts; especially for grid energy storage and supporting energy harvested from renewables).
Prof. Amartya Mukhopadhyay
