Arthritis causes joint pain that flares up during inflammation. This invention is a special injectable gel that stays in the joint and releases its pain-relief medicine only when inflammation occurs. Tiny carriers loaded with a common arthritis drug are mixed into a gel that forms right inside the body. When enzymes rise during a flare-up, they trigger the carriers to break down and free the drug. Lab tests show the gel lasts up to a week, is easy to inject, and works only when needed offering safer, longer-lasting relief.
Millions of people, especially the elderly, suffer from arthritis—a painful joint condition that gets worse with movement and inflammation. Most medicines either wear off too quickly or have serious side effects when taken regularly. There is a need for a better, safer way to manage joint pain that works only when it's actually needed and stays active for a longer time.
- Releases Medicine Only at Pain Sites: The gel holds drug-filled carriers that stay put until inflammation enzymes trigger release.
- Combines Tiny Carriers with an In-Body Gel: Drug carriers sit inside a gel that forms after injection, keeping medicine where it’s needed.
- Gives Up to One Week of Relief: The gel slowly lets out medicine over seven days, cutting down repeat injections.
- Easy to Inject: It flows through common 20–21 gauge needles without clogging or extra force.
- Uses Safe, Natural Materials: It’s built from algae- and shell-derived polymers and a standard arthritis drug.
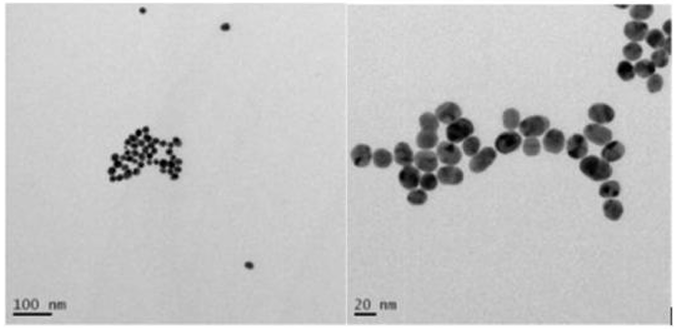
Lab-scale samples were made by mixing drug, fat-like carriers, and polymer solutions. Micelles (18 nm size) were confirmed by electron microscopy. Gels formed instantly when mixed and held micelles in place. In vitro tests measured enzyme-triggered drug release, gel strength, degradation in saline, and cell safety—all demonstrating proof of concept.
The technology has been successfully tested in the lab using safe and commonly available materials. The prototype works well in controlled conditions, showing reliable drug release only when inflammation is present. It has been evaluated for safety, injectability, and effectiveness in test setups.
3
By delivering drug only during painful flares, this gel could greatly improve quality of life for arthritis sufferers. It may reduce overall medication doses, lower side-effect risks, and cut healthcare visits for injections. Elderly and active adults alike could benefit from longer relief and fewer injections, easing the burden on patients and caregivers.
- Targeted joint pain relief in orthopedic care
- Injectable therapies in rheumatology clinics
- Sustained drug delivery platforms in pharmaceutical development
- Biocompatible biomaterials for medical device manufacturing
- Enzyme-triggered systems in smart drug formulation
Geography of IP
Type of IP
202221058551
549372