The invention provides kaolin-supported zerovalent iron (K-nZVI) and sulfidated zerovalent iron (K-SnZVI) composites synthesized through a simple one-step chemical reduction method. These composites offer enhanced removal of hexavalent chromium [Cr(VI)] from groundwater. By combining naturally abundant kaolinite with nanoscale iron and sulfur species, the materials exhibit strong adsorption and partial reduction capabilities. The process avoids the need for anaerobic incubation or pH control and shows high Cr(VI) removal efficiency across a wide range of initial concentrations and geochemical conditions, making it suitable for large-scale water treatment applications.
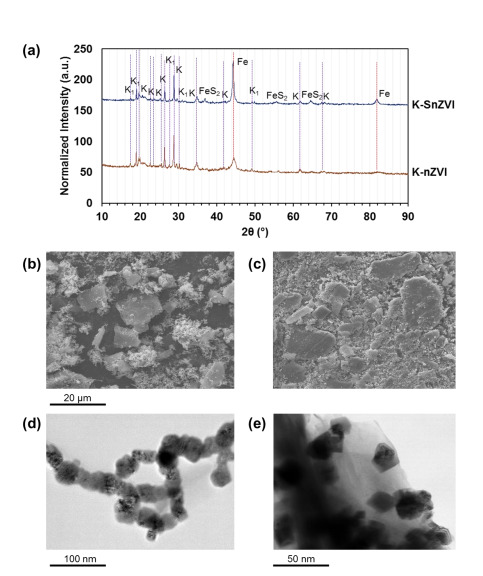
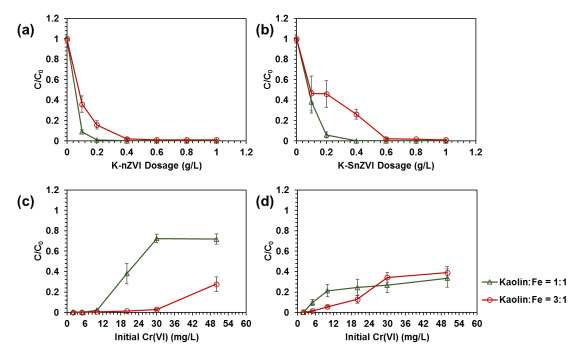
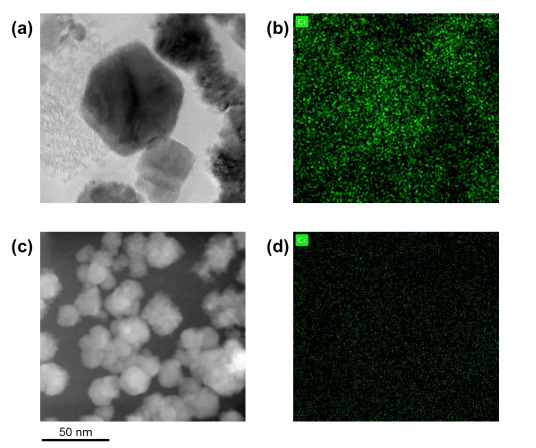
Figure (1) (a) XRD analysis of K-nZVI and K-SnZVI, (b, c) SEM, and (d, e) TEM images of (b, d) K-nZVI and (c, e) K-SnZVI; (2) Removal of Cr(VI) with (a, b) the dosage of 1:1 and 3:1 (a) K-nZVI and (b) K-SnZVI; and (c, d) initial concentrations of dissolved Cr(VI) by (c) K-nZVI and (d) K-SnZVI; (3) TEM images of (a) K-nZVI and (b) K-SnZVI particles after equilibration experiment; TEM-associated EDX area mapping of Cr on the reacted (b) K-nZVI and (d) K-SnZVI.
Chromium (VI) contamination in groundwater is a significant environmental and public health concern due to its toxicity, mobility, and persistence. Traditional remediation techniques using nanoscale zerovalent iron (nZVI) or sulfidated nZVI (SnZVI) face limitations related to cost, stability, and complex synthesis protocols. Additionally, while kaolinite is a low-cost and naturally abundant adsorbent, its adsorption kinetics are slower and less efficient. Thus, there exists a pressing need for a cost-effective, efficient, and scalable method for removing Cr(VI) from contaminated water.
- One-Step Synthesis Method: Kaolin-modified nZVI (K-nZVI) and sulfidated nZVI (K-SnZVI) are synthesized through a single-step chemical reduction process that does not require complex instruments or anaerobic conditions.
- Enhanced Remediation Efficiency: The material demonstrates high Cr(VI) removal efficiency, even at concentrations 20 to 100 times above safe limits, and remains effective over a broad pH range (3–8.3) and diverse geochemical environments.
- Material Composition and Sustainability: The incorporation of naturally abundant kaolinite significantly lowers the amount of iron required, improving sustainability and making the process economically viable.
- Robust Performance: The system maintains consistent Cr(VI) removal performance in the presence of commonly occurring groundwater ions such as chloride, sulfate, nitrate, magnesium, and calcium.
- Faster Kinetics and Adsorptive Capacity: Cr(VI) removal by the composite follows second-order reaction kinetics and is best described by the Freundlich adsorption isotherm, indicating strong and multilayer adsorption behavior.
The prototypes of kaolin-supported zerovalent iron (K-nZVI) and sulfidated zerovalent iron (K-SnZVI) composites were synthesized via a single-step aqueous-phase chemical reduction method. In this process, kaolinite was mixed with FeCl3·6H2O and reduced using NaBH4, with Na2S2O4 used for sulfidation in the case of K-SnZVI. The composites were prepared without the need for inert atmosphere or pH regulation. The synthesized materials were thoroughly characterized using techniques including X-ray diffraction (XRD), scanning and transmission electron microscopy (SEM, TEM), energy-dispersive X-ray spectroscopy (EDX), Brunauer–Emmett–Teller (BET) surface area analysis, and X-ray photoelectron spectroscopy (XPS). Batch experiments confirmed high Cr(VI) removal efficiency, validating the effectiveness of the prototype for practical applications in groundwater remediation.
The K-nZVI and K-SnZVI composites have been successfully synthesized and validated through laboratory-scale batch experiments. Characterization using XRD, SEM, TEM, EDX, BET, and XPS confirmed the structural, morphological, and surface properties of the materials. The composites demonstrated excellent Cr(VI) removal performance under varied pH and geochemical conditions using both ultrapure and synthetic groundwater. The synthesis process is scalable, does not require anaerobic conditions or pH adjustment, and has reached a Technology Readiness Level (TRL) of 4–5, indicating readiness for pilot-scale implementation.
4
This technology provides a cost-effective and scalable solution for the removal of toxic hexavalent chromium [Cr(VI)] from groundwater, addressing a major public health and environmental concern. By using naturally abundant kaolinite and eliminating the need for complex synthesis or operational conditions, the composite ensures accessibility in both urban and rural settings. It supports the provision of safe drinking water in chromium- contaminated regions and contributes to improved environmental quality and long-term community health.
- Groundwater remediation: The composite effectively removes Cr(VI) from contaminated groundwater across a wide range of pH and ionic conditions.
- Industrial effluent treatment: The material can be applied to treat chromium-rich effluents from industries such as electroplating, leather tanning, and metallurgy.
- Environmental engineering consultancies: The technology can be deployed by consultants for site-specific remediation solutions requiring low-cost and scalable treatment options.
- Mining discharge management: The composite is suitable for treating heavy metal-laden runoff and tailing effluents generated from mining operations.
- Rural drinking water infrastructure: Its ease of synthesis and low-cost materials make it ideal for decentralized water purification systems in rural and resource-limited areas.
Geography of IP
Type of IP
202321048337
566414