A novel in-situ self-gelling drug delivery system is developed for localized treatment of glioblastoma multiforme. It comprises transferrin-targeted, dual-drug-loaded lipid nanoparticles embedded in a biocompatible, temperature and ion-responsive hydrogel matrix. This platform ensures sustained, site-specific release and overcomes the limitations of existing therapies like systemic chemotherapy and Gliadel® wafers.
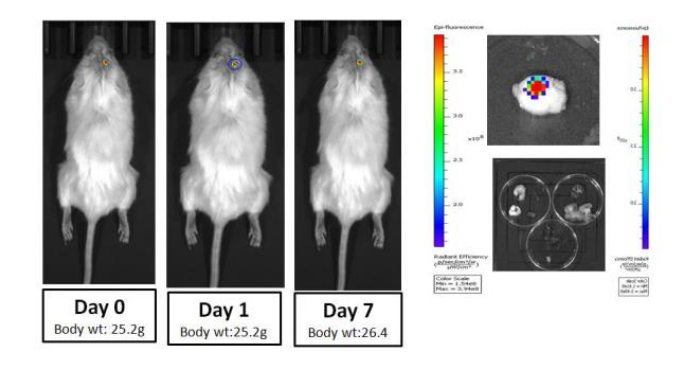
Figure 1. CLSM images showing in-vivo brain penetration analysis of intracranial hydrogel (5µL) embedded with Rh6G loaded targeted lipid nano-vesicles in tumor free mice (G) on day 7th post treatment (Nuclei stained with DAPI, Rhodamine-6G labeled targeted lipid nanovesicles embedded into the HG, Scale bar 20µm).
Glioblastoma multiforme (GBM) is a highly lethal brain tumor with poor prognosis and frequent recurrence despite multimodal treatment. Existing therapies like systemic chemotherapy and Gliadel® wafers suffer from limitations such as poor diffusion, systemic toxicity, high cost, invasiveness, and poor patient outcomes.
- In-Situ Injectable Hydrogel System: The system remains in liquid form at room temperature and transforms into a gel at physiological temperature (37°C), allowing for easy injection through a fine needle and precise filling of irregular post-surgical brain cavities.
- Dual Drug Synergy: The co-encapsulation of temozolomide and miltefosine provides a synergistic therapeutic effect, enhancing anti-tumor efficacy while reducing the required dosage and associated side effects of individual drugs.
- Transferrin Targeting: The lipid nanoparticles are surface-functionalized with transferrin, enabling targeted delivery to glioma cells through receptor-mediated endocytosis, which improves cellular uptake and retention at the tumor site.
- Sustained Release: The combination of the hydrogel matrix and lipid bilayer acts as dual diffusion barriers, ensuring a controlled and prolonged release of therapeutic agents over several days to weeks, thereby maintaining therapeutic levels at the site of action.
- Injectable and Deformable Matrix: Unlike rigid solid implants such as Gliadel® wafers, the hydrogel conforms to the contours of the surgical cavity and remains localized, reducing the risk of migration and associated complications.
- Biodegradable and Biocompatible Components: Composed of safe and degradable polymers, the system eliminates the need for surgical retrieval and minimizes inflammation or immune response, ensuring patient safety and comfort.
The prototype consists of transferrin-functionalized, dual-drug-loaded lipid nanocarriers embedded in a thermosensitive and ionically crosslinked amphiphilic hydrogel (gellan-HPMC-PCL). Tested successfully in in vitro and murine GBM models with high drug encapsulation efficiency and retention.
The technology is currently in the late preclinical stage, with studies being conducted in compliance with Good Laboratory Practices (GLP), including extensive animal testing.
5
This technology offers a minimally invasive, cost-effective, and targeted post-surgical therapeutic platform for GBM, potentially improving survival rates and quality of life by reducing recurrence and systemic side effects.
- Drug Delivery Systems: Enables localized, sustained release of drugs at the tumor site
- Cancer Treatment Technologies: Offers effective combination therapy for aggressive brain tumors
- Medical Devices for Neurosurgery: Injectable gel used post-surgery to prevent tumor recurrence
- Targeted Therapy Platforms: Transferrin-tagged nanoparticles ensure precise tumor targeting
Geography of IP
Type of IP
202021009092
408307