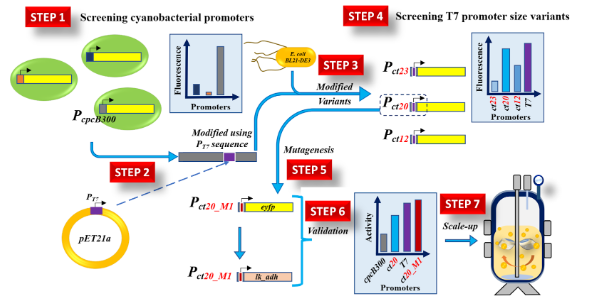
This invention presents a recombinant promoter system for inducer-free protein expression in E. coli. The promoter is designed by fusing cyanobacterial cpcB300 promoter sequences with truncated T7 promoter fragments, resulting in hybrid constructs that drive strong, constitutive expression. Among the variants tested, ct20-M1 hybrid promoter outperformed the standard T7 system, delivering higher protein yields in both shake flask and bioreactor settings. The system eliminates the need for chemical inducers, offering a cost-effective and scalable solution for applications in biopharmaceuticals, industrial enzymes, and synthetic biology.
Conventional recombinant protein expression systems, such as the IPTG-inducible T7 system, are costly, complex, and metabolically burdensome due to the need for inducer molecules, precise timing of induction, and intensive process monitoring. These limitations hinder scalability, automation, and industrial feasibility–especially when producing multiple proteins in parallel.
- Inducer-Free Expression: The system eliminates the need for chemical inducers such as IPTG by enabling constitutive protein expression. This significantly reduces operational costs and simplifies upstream processing by removing the requirement to monitor cell growth phase or precisely time the induction step.
- Engineered Hybrid Promoters: The promoters are constructed by fusing fragments of the cyanobacterial cpcB300 promoter with size-optimized sequences from the T7 promoter. This rational design approach enhances transcriptional strength, resulting in synthetic promoters that outperform both native cpcB300 promoter and conventional T7-based inducible systems.
- Improved Yield and Efficiency: Among the tested constructs, the ct20-M1 promoter variant achieved the highest expression levels, delivering up to 1.3 times more protein than the IPTG-induced T7 system under equivalent conditions. This was demonstrated in both shake flask and bioreactor-scale experiments using eYFP and LkADH as model proteins.
- Versatile Application: The promoter system supports expression of a broad range of recombinant proteins, including fluorescent reporters and industrial enzymes. It functions across different media types and cultivation conditions, making it applicable to both research laboratories and commercial production facilities.
- Scalable and Automation-Friendly: The technology has been validated under high-cell-density growth conditions in 3L bioreactors, maintaining stable protein production without inducer intervention. Its ability to function without precise timing or process adjustments makes it ideal for integration into automated and continuous biomanufacturing platforms.
The prototype system consists of genetically engineered recombinant promoters constructed by fusing truncated sequences of the T7 promoter (ranging from 8 to 20 base pairs) into specific regions of the cyanobacterial cpcB300 promoter. These hybrid promoter constructs were designed to enable constitutive, inducer-free expression of target proteins in E. coli. The promoters were cloned into expression vectors upstream of genes coding for either fluorescent proteins (eYFP) or functional enzymes (LkADH). Among the tested variants, ct20-M1 promoter showed the highest transcriptional strength.
The engineered plasmids were transformed into E. coli cells, and protein expression was evaluated in both shake flask and 3-liter bioreactor setups, using both complex and defined media. Fluorescence assays and SDS-PAGE analysis confirmed successful expression of the target proteins. The ct20-M1 promoter consistently outperformed the IPTG-inducible T7 promoter under equivalent conditions, validating its potential for cost-effective, scalable, and inducer-free protein production across different bioprocessing environments.
The recombinant promoter system has been successfully developed, cloned, and validated in laboratory settings. Expression strength has been quantitatively tested in shake flask and 3L bioreactor studies. Among the tested variants, ct20-M1 promoter was identified as the most promising and is fully functional in both rich and defined media. The technology is at a mature prototype stage and demonstrates strong readiness for industrial scale-up and application in biotech manufacturing.
4
This invention offers a cost-effective and scalable alternative to traditional chemically induced protein expression systems. By eliminating the need for expensive inducers and simplifying the cultivation process, it makes recombinant protein production more economically and environmentally sustainable. The technology is especially valuable in biopharmaceuticals, industrial enzyme production, and synthetic biology, where consistent and high-yield expression is critical. It supports automation-friendly workflows, reduces operational complexity, and enhances access to protein production platforms in low-resource or decentralized settings.
- Recombinant protein production for industrial enzymes
- Biopharmaceutical manufacturing (e.g., therapeutic proteins, vaccines)
- Synthetic biology and metabolic engineering platforms
- Academic and research laboratories
- Automated and continuous bioprocessing systems
Geography of IP
Type of IP
202221003116
548180