This innovation is a simple, cost-effective, clean-room free fabrication method for polymeric microneedle patches using CNC-machined stainless steel molds and PDMS-based soft lithography. This process offers a robust and reproducible alternative to expensive MEMS-based microfabrication and enables mass production using biodegradable polymers for drug and vaccine delivery applications.
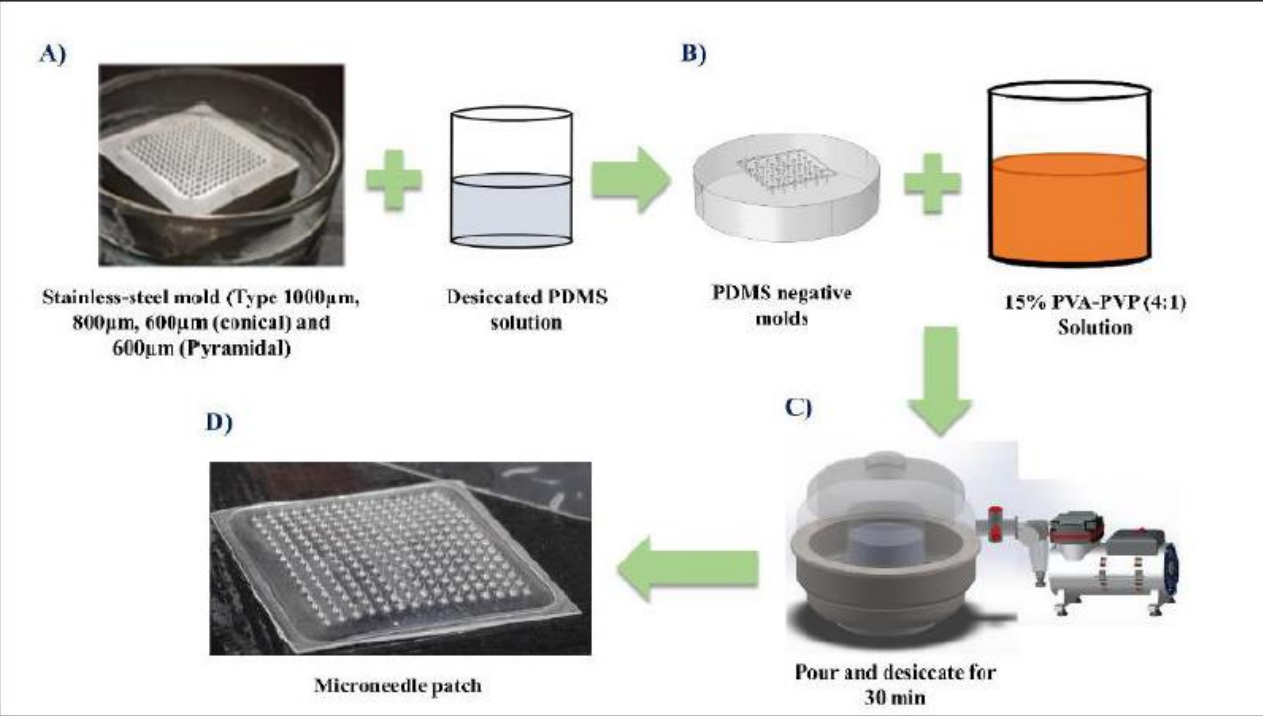
Figure (1) Graphical representation of the fabrication process of PVA-PVP MNs: (1a) PDMS was poured on the stainless-steel mold, (1b) The polymer solution was poured on the PDMS negative mold, (1c) Desiccation with the help of a vacuum pump to drive the polymeric solution's entry into the microneedle cavities, (1d) Polymeric microneedle patch; (2) Applying for ethical approval for benzoyl peroxide patches for acne treatment at ATREC hospital
Conventional microneedle fabrication methods often require expensive clean-room environments and complex MEMS technologies, making them inaccessible for widespread applications in drug and vaccine delivery.
- Clean-Room Free Fabrication: This technology eliminates the requirement for clean-room facilities by utilizing a CNC-machined stainless-steel mold along with PDMS-based soft lithography for microneedle fabrication.
- Low-Cost and Reproducible Process: The process involves a one-time fabrication of a master mold, which can be reused multiple times for casting, thereby significantly reducing overall production costs and ensuring high reproducibility.
- Material Versatility: The method is compatible with a wide range of soft biodegradable polymers such as PVA, PVP, PCL, and PLGA, allowing flexibility in material selection and enabling precise control over drug release profiles.
- High Mechanical Strength: The fabricated microneedles demonstrate a failure force greater than 0.24 N per needle, which is sufficient to ensure effective penetration into the skin without any risk of fracture.
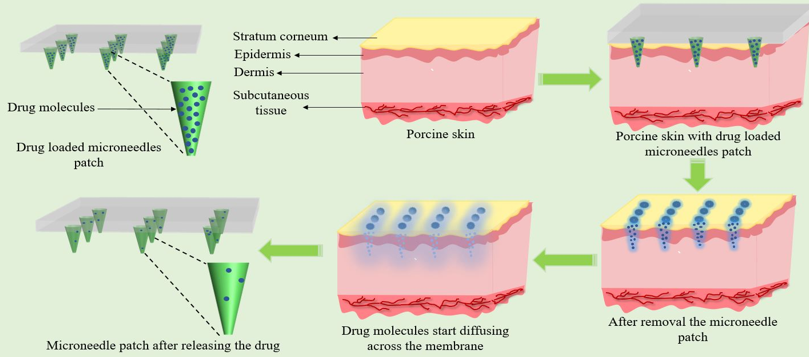
A prototype patch with 225 microneedles (15×15 array) using PVA/PVP (4:1) was fabricated via vacuum-assisted mold filling and drying at 37°C. It was tested for insertion in agarose gel, drug release through dialysis membranes, and penetration in goat skin.
The technology is currently between the lab-scale and prototype stages of development.
3
This clean-room free, affordable microneedle platform democratizes access to minimally invasive transdermal delivery systems, especially valuable in rural, low-income, or resource-constrained healthcare setups. It supports mass vaccination, painless therapeutic delivery, and potential home use.
- Healthcare and Medical Devices: Used for painless, minimally invasive delivery of therapeutics, improving patient compliance and accessibility in clinical and home-care settings
- Transdermal Drug Delivery: Enables efficient delivery of drugs through the skin by bypassing the gastrointestinal tract and reducing side effects associated with oral or injectable routes
- Vaccine and Gene Delivery: Provides a needle-free platform for delivering vaccines and genetic materials directly into the dermis or epidermis, which are rich in immune-responsive cells
- Biosensing and Bioimaging: Microneedles can serve as tools for extracting interstitial fluids or detecting biological markers, making them suitable for wearable diagnostics and real-time health monitoring
- Pharmaceutical Manufacturing: Supports scalable production of drug-loaded patches, suitable for commercial manufacturing of advanced drug delivery systems
Geography of IP
Type of IP
202221034506
544751