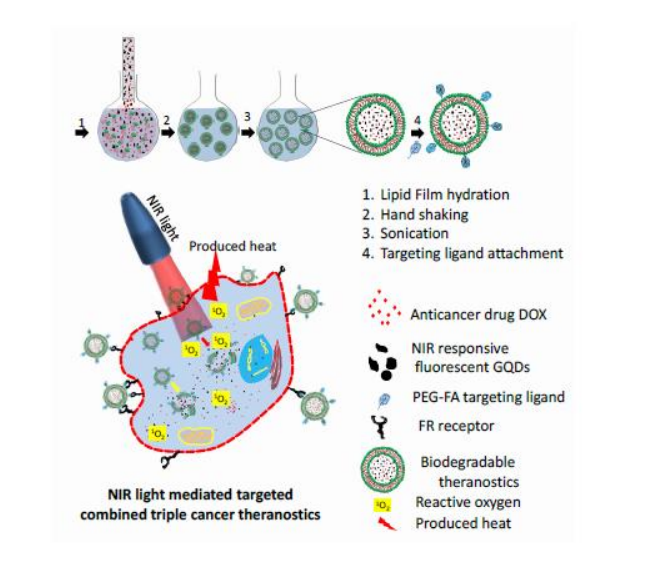
The invention presents a biodegradable fluorescent liposomal nanocomposite system for targeted cancer therapy. The platform integrates liposomes with graphene quantum dots (GQDs) and encapsulates the anticancer drug Doxorubicin (DOX). Functionalization with folic acid (FA) ensures selective delivery to folate receptor-expressing cancer cells. This smart nanocomposite enables multimodal imaging and combined chemotherapy, photothermal, and photodynamic therapy, triggered under near-infrared (NIR) light. It offers excellent aqueous stability, biocompatibility, photostability, and NIR-triggered drug release and degradation. The technology holds strong potential for personalized, image-guided, and minimally invasive cancer treatment.
Figure 1. Schematic showing the preparation, surface functionalization and multifunctional targeted theranostics ability of nanohybrid. Distribution of drug and GQDs and degradation of nanohybrid in cancer cell interior is seen from schematic. The yellow arrow indicates the nanohybrid degradation in the endosome environment whereas the red arrow shows the degradation during NIR light exposure.
Current theranostic systems based on metal quantum dots or organic dyes suffer from toxicity, poor degradation, low photostability, and limited therapeutic efficacy. There is an unmet need for a biodegradable, biocompatible, and multifunctional nanoplatform that enables targeted drug delivery and image-guided triple therapy, especially for aggressive cancers.
- Biodegradable & Biocompatible System: The technology utilizes liposome-based carriers embedded with graphene quantum dots (GQDs), which ensure safe biodegradation and minimal toxicity, making the system suitable for biomedical use.
- Triple Therapeutic Action: The nanocomposite platform enables a combination of chemotherapy, photothermal therapy, and photodynamic therapy, offering a comprehensive and synergistic approach to cancer treatment.
- NIR-Responsive Imaging & Therapy: The embedded graphene quantum dots are responsive to near-infrared (NIR) light, allowing for targeted imaging and light-triggered therapeutic action within cancerous tissues.
- Targeted Drug Delivery: The surface of the nanocomposite is functionalized with folic acid (FA), enabling selective targeting of cancer cells that overexpress folate receptors, thereby enhancing therapeutic specificity.
- Stimuli-Responsive Drug Release: The system is designed to release the encapsulated anticancer drug in response to acidic conditions commonly found in tumor environments and upon exposure to NIR light, allowing precise and controlled drug delivery.
Lab-scale prototypes of NFGL and DOX-NFGL-FA were synthesized, functionalized, and characterized using TEM, SEM, AFM, UV-Vis, Raman, DLS, FTIR, and fluorescence spectroscopy. It was tested on MCF-7 breast cancer cell lines for uptake, ROS generation, and therapeutic response. Drug loading efficiency, degradation, and photothermal effects were confirmed under NIR light and pH-responsive conditions.
This technology is developed at laboratory scale and tested for cancer therapy and imaging.
3
This innovation offers a non-invasive, localized, and targeted solution for cancer treatment. It minimizes off-target effects, reduces systemic toxicity, and enhances therapeutic outcomes, thus improving patient quality of life. It can play a transformative role in precision medicine and next-generation cancer care.
- Biomedical Engineering: Nano-bio interface and theranostic systems
- Cancer Theranostics: Diagnosis, imaging, and therapy
- Pharmaceutical Industry: Smart drug delivery systems
- Medical Devices: NIR-based imaging and therapy devices
- Nanotechnology and Materials Science: Biodegradable nanocomposites
Geography of IP
Type of IP
201821020431
372447