This invention presents a targeted cancer therapy using NIC-HA micelles, a nanoconjugate of niclosamide anchored to hyaluronic acid. These ~200 nm micelles exhibit selective uptake and strong growth inhibition in CD44-overexpressing cancer and stem cells. Unlike antibody or peptide-based therapies, NIC-HA shows high biocompatibility (~95%) and hemocompatibility (~99%), addressing toxicity and cost concerns. In vitro and in vivo studies (HT1080 tumor xenograft) demonstrated significant tumor suppression (60%, p < 0.05) and cancer cell death, primarily through STAT-3 pathway inhibition and apoptosis in triple-negative breast cancer cells.
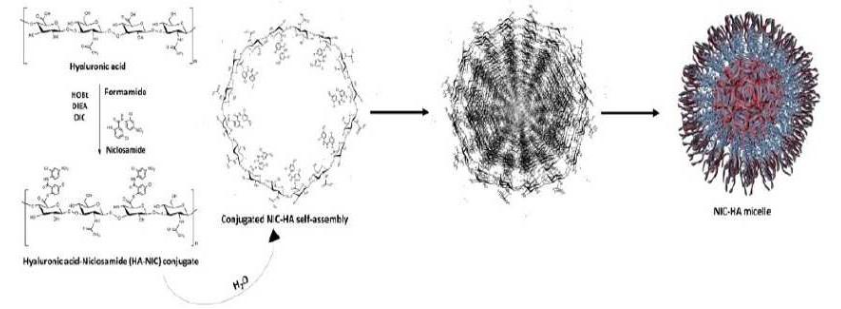
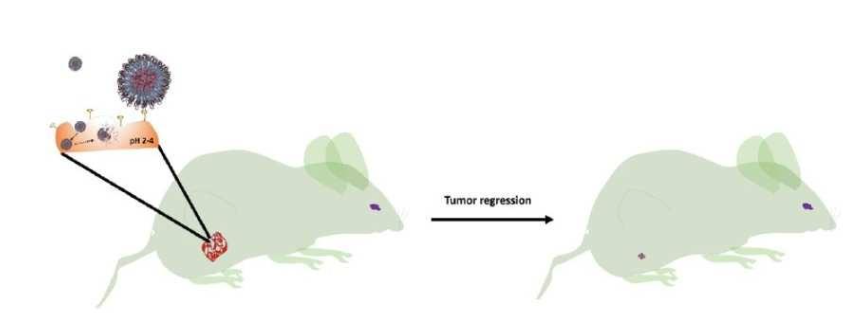
Figure (1) Schematic representation of covalent conjugation of NIC-HA followed by its self-assembly in aqueous medium; (2) Anti-tumor activity of designed NIC-HA nanoconjugate for site-selective tumor regression; Publication: Nishant Kumar Jain, Shalini Dimri, Rajendra Prasad, Abhijit De and Rohit Srivastava, Characteristics of Molecularly Engineered Anticancer Drug Conjugated Organic Nanomicelles for Site-Selective Cancer Cell Rupture and Growth Inhibition of Tumor Spheroids, ACS Appl. Bio Mater., 2020, 3, 10, 7067
Targeted cancer therapy faces several limitations including systemic toxicity, high cost, limited tumor selectivity, and complex synthesis protocols of existing drug delivery systems such as antibody- or peptide-based therapies. There is a need for a simpler, cost-effective, and biocompatible nanocarrier system that ensures site-selective drug delivery, enhanced cellular uptake, and effective tumor regression. The lack of scalable, reproducible, and stable methods further hampers clinical translation. Addressing these challenges, the present invention aims to develop self-assembled niclosamide-hyaluronic acid (NIC-HA) conjugated micelles with high biocompatibility, tumor-specific uptake, and therapeutic efficacy, especially against CD44+ cancer cells.
- Simple and Self-Assembled Design: The NIC-HA system forms stable micelles through a straightforward self-assembly process, eliminating the need for complex formulation steps.
- Ambient and Scalable Synthesis: The synthesis and purification are carried out at room temperature using simple protocols, offering high reproducibility and scalability for industrial production.
- Stable and Uniform Nanostructures: The resulting micelles are spherical, monodispersed (~200 nm), and exhibit a negative zeta potential, promoting physical stability and passive targeting.
- High Biocompatibility and Hemocompatibility: NIC-HA micelles show ~95% biocompatibility and ~99% hemocompatibility, ensuring safe systemic application.
- Targeted Delivery to Tumor Cells: The system enables site-selective uptake in CD44-overexpressing tumor cells and cancer stem cells, enhancing therapeutic precision.
- Controlled Bioactivity via Linkage Type: The amide-linked NIC-HA shows reduced potency compared to free niclosamide, while the ester-linked NIC-HA improves apoptosis, cellular uptake, CD44+ targeting, and migration inhibition.
- Molecular-level Targeting: Treatment with NIC-HA leads to concentration-dependent suppression of activated STAT-3 signalling, promoting apoptosis in cancer cells.
- Reduced Tumor-Initiating Potential: The NIC-HA conjugate significantly lowers mammosphere formation, indicating reduced stemness and recurrence potential.
- Effective In Vivo Performance: NIC-HA shows up to 60% tumor regression in animal models while maintaining body weight, validating its therapeutic efficacy and safety.
The present invention provides Niclosamide-Hyaluronic Acid (NIC-HA) conjugated micelles, where niclosamide is anchored to hyaluronic acid via ester or amide linkages. The ester-linked NIC-HA micelles are synthesized by dissolving hyaluronic acid in preheated formamide to form a 0.5% w/v solution, followed by sequential addition of hydroxy benzotriazole (HOBt), N,N-diisopropylethylamine (DIPEA), and N,N′-diisopropylcarbodiimide (DIC) while cooling the mixture. A solution of niclosamide in dimethylformamide (DMF) is then added, and the mixture is dialyzed against DMF using a dialysis membrane. The dialyzed solution is lyophilized to obtain the NIC-HA conjugate, which is then dispersed in water to allow micellar self-assembly into monodisperse nanospheres.
For the amide-linked NIC-HA nanoconjugates, the process begins with the reduction of niclosamide’s nitro group using zinc powder in an acidic medium. The reduced product is dissolved in a 1:1 mixture of acetone and ethanol, heated at 96°C, and further reacted with zinc and hydrochloric acid. After cooling and filtering, the resultant solution is added to an aqueous HA solution pre-activated with 1-(3-dimethyl aminopropyl)-3-ethylcarbodiimide (EDC) and N-hydroxysuccinimide (NHS). The final product is dialyzed against water and lyophilized to yield the amide-bonded NIC-HA nanoconjugate.
Technology is developed at laboratory scale and evaluated in vitro models.
3
The invention offers a targeted, safer, and cost-effective approach to cancer treatment, potentially reducing the side effects and financial burden of conventional chemotherapy. By improving drug delivery specifically to tumor cells, it may enhance patient outcomes, increase treatment accessibility, and lower recurrence rates. This can contribute to better quality of life for cancer patients and more efficient use of healthcare resources.
- Pharmaceutical industry
- Biotechnology industry
- Nanomedicine
- Healthcare
- Oncology
- Biomedical research
- Contract research organizations (CROs)
Geography of IP
Type of IP
202021038024
429445