The present invention relates a process for preparing biocompatible porous silica nanoparticles, which comprises preparing an aqueous solution of at least one lipid, adding a base to the aqueous solution to attain the pH in the range of 10 to 12 to obtain a basic solution of the lipid, adding at least one silica source to the basic solution of said lipid under stirring to obtain a reaction mixture, precipitating the reaction mixture by stirring to obtain a precipitate and isolating followed by drying the precipitate to obtain the biocompatible porous silica nanoparticles. The process is simple, conducted at room temperature, and avoids toxic surfactants, resulting in stable and safe silica nanoparticles.
There is a need for a simple, room-temperature process to synthesize biocompatible mesoporous silica (MS) nanoparticles that overcome the limitations of existing method, such as toxicity from CTAB surfactants, poor stability, premature drug release, low drug loading capacity, large particle size, and complex multistep synthesis.
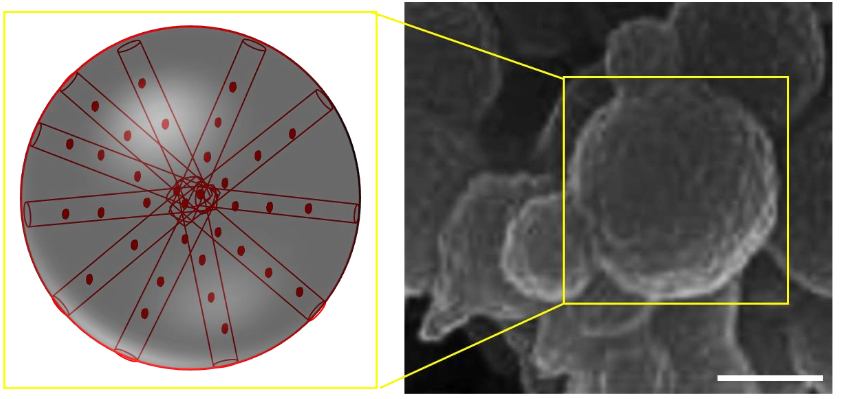
- Uniform Nanoparticle Size and Morphology: The synthesized porous silica nanoparticles exhibit a uniform spherical shape with a controlled particle size of approximately 150–200 nm, confirmed by TEM and SEM imaging.
- Well-Ordered Mesoporous Structure: The material demonstrates a well-ordered two-dimensional hexagonal mesoporous structure, as evidenced by a sharp and intense peak in low-angle powder X-ray diffraction (LAPXRD).
- High Biocompatibility: The use of a biocompatible lipid surfactant ensures high cell viability (>95%) even at elevated nanoparticle concentrations (200 μg/mL).
The process for preparing biocompatible porous silica nanoparticles involves first preparing an aqueous solution of at least one lipid, such as DSPC, DPPC, DMPC, or POPC. A base such as NaOH is then added to adjust the pH to 10–12, forming a basic lipid solution. A silica source like tetraethyl orthosilicate (TEOS) is added under stirring to form a reaction mixture. This mixture is stirred to allow precipitation, yielding a silica nanoparticle precipitate. The precipitate is then isolated and dried to obtain the final nanoparticles. The process operates at room temperature, maintains the ordered 2D structure of the lipid throughout, and results in spherical particles with a size range of 50–200 nm. The optimized molar ratios of lipid to silica and silica to base ensure structural integrity and biocompatibility.
Technology has been developed at the laboratory scale.
3
The invention enables the safe and scalable production of biocompatible porous silica nanoparticles, advancing targeted drug delivery and diagnostic imaging. It reduces dependence on toxic surfactants, ensuring safer nanomedicine applications. This promotes more effective and patient-friendly treatments for complex diseases like cancer.
- Pharmaceutical industry
- Biotechnology industry
- Nanomedicine industry
- Healthcare industry
- Medical devices industry
- Diagnostic imaging industry
Geography of IP
Type of IP
202021025105
401827