The invention relates to a novel EC-free sulfone-based electrolyte comprising 1 M LiPF₆ in EMS:DMC (3:7), offering superior high-voltage stability (>4.6 V), lower impedance (~3.6 Ω), and ~87% capacity retention after 50 cycles with NMC811 cathodes in Li half-cells. It enhances battery lifespan and safety under high-voltage cycling.
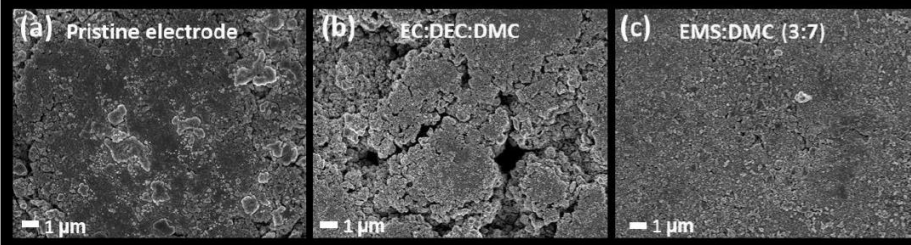
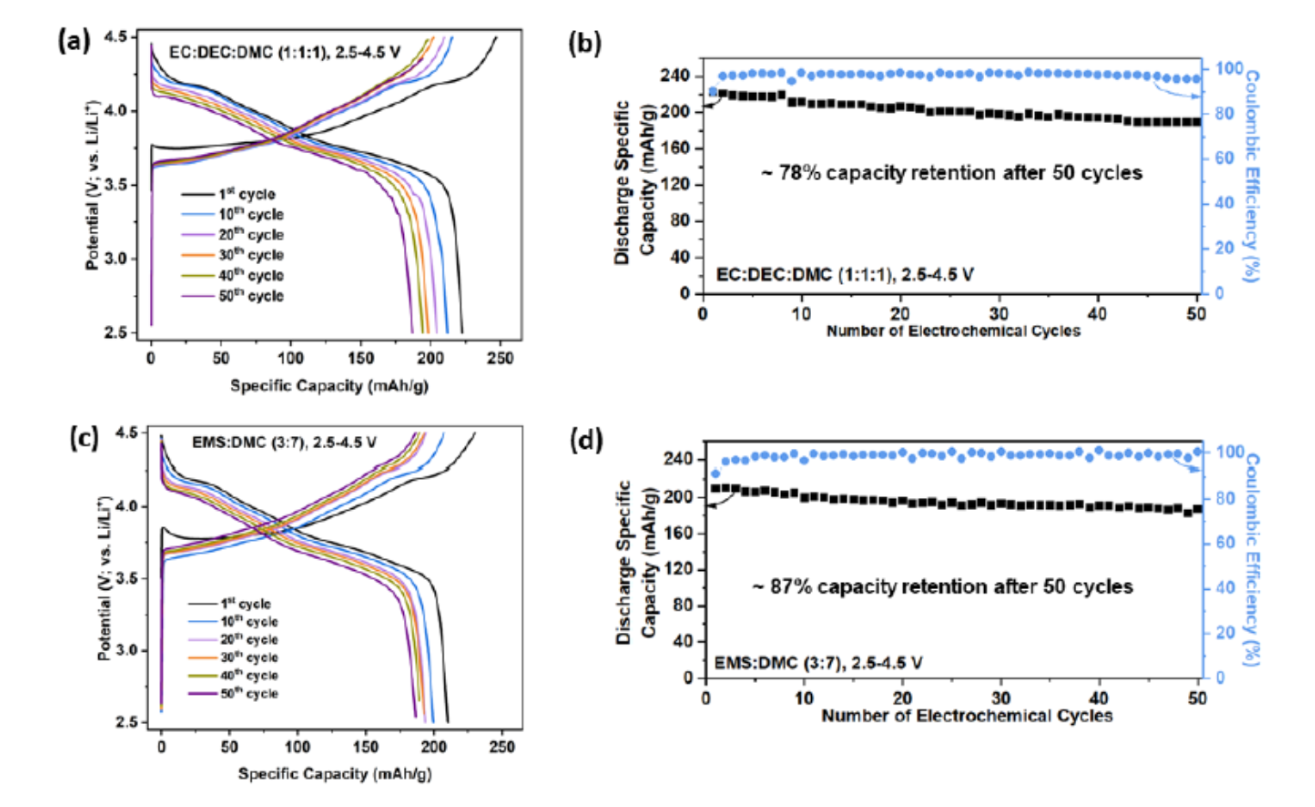
Figure (1) SEM images of the Li-NMC811 electrodes (1a) in pristine condition (i.e., before cycling) and (1b, 1c) after being galvanostatically cycled 50 times in Li ‘half’ cells @ C/10 using the (1b) ‘standard’ carbonate-based EC:DEC:DMC 1:1:1 and (1c) newly-developed sulfone-based EMS:DMC (3:7) electrolytes; (2) Potential profiles and electrochemical cycling performance of Li-NMC811 in Li ‘half’ cells at current densities equivalent to C/10 using the the respective electrolytes (viz., the ‘standard’ and the newly-developed ones); as per (2a) Potential profiles and (2b) Cycling stability (i.e., variation of discharge capacity with electrochemical cycle number) and coulombic efficiency (CE) up to 50 galvanostatic cycles using the ‘standard’ carbonate-based (1M LiPF6 containing) EC:DEC:DMC 1:1:1 electrolyte; (2c) Potential profiles and (2d) Cycling stability and CE up to 50 galvanostatic cycles using the newly-developed sulfone-based (1M LiPF6 containing) EMS:DMC (3:7) electrolyte.
Current lithium-ion battery (LIB) electrolytes based on carbonate solvents suffer from poor oxidative stability beyond 4.3 V, limiting compatibility with high energy-density Ni-rich cathodes such as LiNi₀.₈Mn₀.₁Co₀.₁O₂ (NMC811). These limitations affect battery safety, cyclic stability, and performance at high voltages.
- High Anodic Stability: Ethyl methyl sulfone (EMS) provides a wide electrochemical stability window of up to 5.8 V, enabling stable electrochemical cycling at high voltages beyond 4.5 V vs. Li+/Li.
- Synergistic Solvent Blend: The use of dimethyl carbonate (DMC) effectively reduces the viscosity of EMS while preserving its high anodic stability, ensuring better ionic mobility.
- Reduced Impedance: The newly developed electrolyte exhibits a low impedance of approximately 3.6 Ω, which enhances ion transport and improves overall battery efficiency.
- Enhanced Cycle Life: The battery demonstrates around 87% capacity retention after 50 charge/discharge cycles at a C/10 rate with a high-voltage cut-off of 4.5 V vs. Li+/Li, indicating superior cyclic stability.
- Structural Stability: Post-cycling SEM analysis confirms that the electrode surface remains intact and free from cracks or damage, highlighting the structural stability of the cathode material when using the new electrolyte.
The electrolyte was tested in CR2032 coin cells assembled with a lithium metal anode and an NMC811 cathode, where the electrolyte was prepared by dissolving 0.7595 g of LiPF₆ in 5 mL of a solvent mixture of ethyl methyl sulfone (EMS) and dimethyl carbonate (DMC) in a 3:7 volume ratio, followed by stirring the solution for 12 hours under inert (argon) atmosphere to ensure uniform dissolution.
4
The technology supports the development of high-energy, safer lithium-ion batteries for electric vehicles and grid storage applications, while also reducing the environmental footprint by enabling the use of cobalt-free cathodes and significantly increasing battery lifespan.
- Electric Vehicles and E-mobility Solutions: Enables safer, high-voltage batteries with longer range and improved performance for EVs and hybrid vehicles
- Consumer Electronics: Supports compact, high-capacity batteries for smartphones, laptops, and wearables with enhanced safety and lifespan
- Renewable Energy Storage Systems: Improves efficiency and durability of battery storage for solar and wind energy integration
- Battery Manufacturing: Offers advanced electrolyte formulation for next-gen Li-ion cells with better thermal and electrochemical stability
- Defense and Aerospace Power Systems: Provides reliable high-density energy storage suited for critical, high-performance applications in defense and space
Geography of IP
Type of IP
202421027220
557929