This invention presents a green, biocatalytic process for producing optically pure (1R,2S)-ephedrine using genetically engineered E. coli expressing the methyltransferase enzyme phenylethanolamine N-methyltransferase (PNMT). The process enables highly selective monomethylation of (1R,2S)-norephedrine under mild conditions, avoiding toxic chemicals and harsh steps. It offers high regio- and enantioselectivity, minimal waste, and easy integration with existing biocatalytic pathways, making it ideal for sustainable pharmaceutical manufacturing.
Existing industrial methods for producing optically active ephedrine involve multistep syntheses using toxic reagents, high energy inputs, and costly chiral resolution. They often show poor regio- and enantioselectivity, resulting in unwanted by-products, high purification costs, and environmental concerns. A greener, more efficient, and selective approach is urgently needed for sustainable synthesis of chiral secondary aromatic amines like ephedrine.
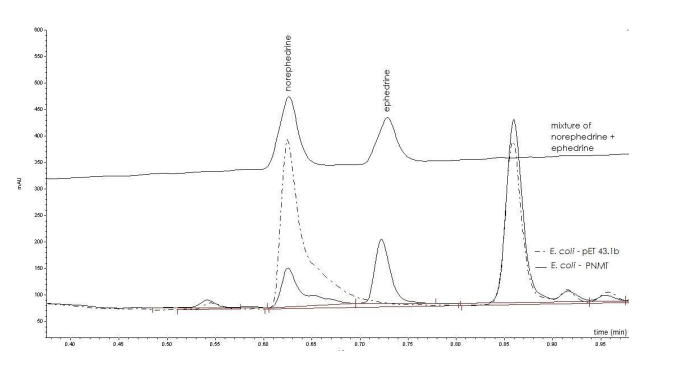
- Biocatalytic Specificity: The process employs Escherichia coli BL21(DE3) genetically engineered to express human phenylethanolamine N-methyltransferase (PNMT), which selectively catalyzes monomethylation of (1R,2S)-norephedrine to (1R,2S)-ephedrine with over 99% enantioselectivity and 100% regioselectivity.
- Sustainable Process: The method uses non-toxic, renewable substrates and co-factors such as S-adenosyl methionine (SAM) generated in situ, avoiding hazardous chemicals like methyl halides or formaldehyde.
- Kinetic Resolution Capability: The enzyme selectively accepts the (1R,2S)-enantiomer from racemic norephedrine mixtures, enabling efficient chiral resolution and reducing the need for downstream separation steps.
- Integration-Ready Design: This biocatalytic step is compatible with upstream enzymatic pathways (e.g., transaminase-mediated synthesis of norephedrine), making it suitable for one-pot or cascade synthesis systems.
- Minimized Waste and Energy Footprint: The mild aqueous reaction conditions (ambient temperature, neutral pH) and selectivity significantly reduce waste, energy usage, and purification overheads.
A genetically engineered E. coli BL21(DE3) strain was developed by cloning the PNMT gene into the high-expression vector pET43.1b. This recombinant strain—designated E. coli-PNMT was cultured and induced under optimized conditions, converting 200-500 μM norephedrine into optically pure (1R,2S)-ephedrine. The reaction was monitored and confirmed using UPLC, LC-MS, and chiral HPLC techniques.
The technology is currently undergoing hypothesis testing and has been demonstrated in laboratory settings and/or animal models to validate its proof of concept.
4
The invention aligns with global trends toward green and sustainable pharmaceutical manufacturing. By enabling the safer production of key active pharmaceutical ingredients (APIs) such as ephedrine, it minimizes both environmental and occupational hazards associated with traditional chemical synthesis. The technology supports indigenous bio-manufacturing capabilities, reducing reliance on expensive and imported chemical reagents. Additionally, it promotes the development of circular and bio-based economies within the pharmaceutical sector, contributing to more sustainable and self-reliant healthcare infrastructure.
- Pharmaceutical Manufacturing: Green synthesis of decongestants, CNS stimulants, and anti-obesity medications
- Synthetic Organic Chemistry: Stereo- and regio-selective functional group transformations
- Industrial Biocatalysis: Enzyme-mediated synthesis of chiral amines and intermediates
- Bioprocess Engineering: Integration into microbial fermentation platforms
- Chiral Intermediates Market: Manufacture of optically pure building blocks for drug synthesis
Geography of IP
Type of IP
201621014963
391816