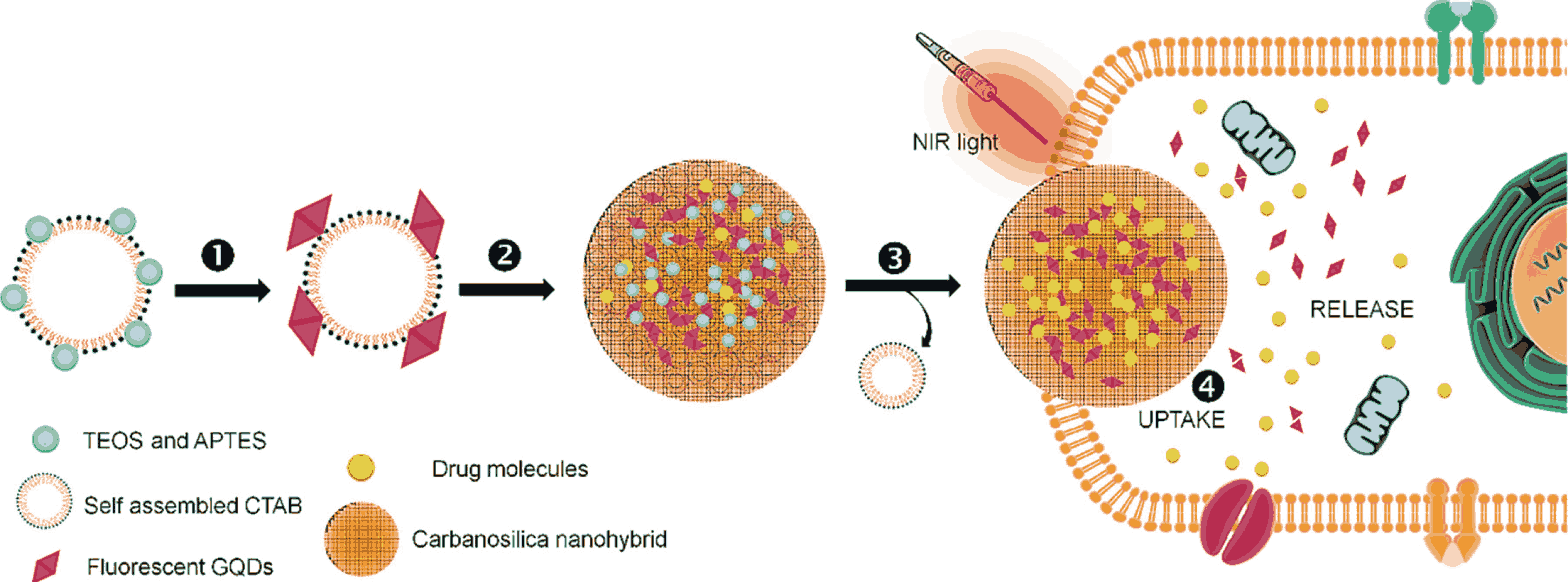
This invention outlines a method to create drug-loaded fluorescent Graphene Quantum Dots (GQDs) embedded in a mesoporous silica structure for imaging and treating solid tumors. The process begins by combining red-emissive GQDs with a surfactant called CTAB and silica precursors. GQDs aid in imaging. The mixture then undergoes hydrolysis and condensation, forming a silica coating around the GQDs attached to the CTAB surfactant. Next, the mixture is centrifuged at high speeds to separate the GQDs embedded silica nanostructures from the rest of the solution. The CTAB surfactant is then removed, leaving behind mesoporous silica nanostructures with embedded GQDs. "Mesoporous" means these structures have tiny pores, which are useful for holding drugs. Finally, these porous structures are loaded with a drug, creating a compound that can be used for both imaging and removing tumors, thus enhancing the effectiveness of solid tumor ablation.
Figure 1. Schematic showing the surfactant mediated sol–gel process of red fluorescent GQDs encapsulated porous silica nanohybrids named as carbanosilica: ① Deposition of silica precursor (a mixture of (3-aminopropyl)triethoxysilane (APTES) and tetraethylorthosilicate (TEOS)) on self-assembled CTAB surfactant, ② decoration of red emissive GQDs on silica coated CTAB assemblies, ③ matured fluorescent GQDs encapsulated porous silica nanohybrids, and ④ surfactant free fluorescent graphene quantum dots encapsulated porous silica nanohybrids uptake into cancer cells during NIR light exposure (2) Scanning electron microscopic (SEM, a) and transmission electron microscopic (TEM, b) images of carbanosilica (graphene quantum dots (GQDs) embedded mesoporous silica). Inset shows the particle size distribution of carbanosilica nanoparticles.
- Existing tumour ablation (removal) techniques are extremely disruptive and cause damage to healthy tissues.
- Although there has been progress in developing Near-Infrared (NIR) light-responsive nanostructures and photo-triggered techniques, current methods face significant limitations including insufficient depth penetration and prolonged retention in tumors, making monitoring difficult.
- Silica, although effective for drug delivery, has low targeting ability, poor tumor accumulation, and slow degradation.
While fluorescent Graphene Quantum Dots (GQDs) are promising for targeted tumor imaging, they suffer from rapid clearance from the body and toxicity.
Therefore, there is a need to address these disadvantages and improve the efficacy of NIR light mediated tumor ablation therapeutics.
- Integration of Fluorescent GQDs within Mesoporous Silica (MS): Embedding fluorescent GQDs within mesoporous silica (MS) leverages GQDs' better fluorescent properties for imaging and MS's large cargo capacity.
- Combined Therapeutic Approach: By integrating imaging and therapeutic capabilities, the invention enables a combinational treatment strategy. The precise imaging guides the photothermal therapy, ensuring that the tumor is effectively targeted and ablated, while simultaneously delivering the therapeutic drug.
- Improved Imaging and Monitoring: Red-emissive GQDs enable deep tissue visualization, allowing precise imaging of the tumor and facilitating accurate monitoring and guidance during NIR light mediated tumor ablation.
- Real-Time Monitoring: The fluorescent properties of the GQDs allow for real-time monitoring of the treatment process. This ensures that the progress of tumor regression can be tracked accurately, providing immediate feedback on the effectiveness of the therapy, and allowing for adjustments as needed.
- Efficient Drug Loading and Delivery: The method involves loading the drug into the MS nanostructure embedded with GQDs, ensuring direct delivery to the tumor site, enhancing therapeutic effectiveness while minimizing side effects.
- Photothermal Therapy: The invention utilizes NIR light to heat the GQDs within the MS structure. This localized heating, known as photothermal therapy, raises the temperature of the tumor region, inducing thermolysis (heat-induced cell death) of the tumor cells.
- Targeted and Controlled Release: The drug-loaded silica nanoparticles allow for controlled release of the loaded drug, ensuring sustained therapeutic agent release at the tumor site, increasing the accumulation of the drug-loaded nanostructure in the tumor, and improving treatment efficacy.
The prototype described is a proof of concept, and has been tested in vitro, with the following results:
- Achieved a high drug loading capacity (~31%) within the carbanosilica nanohybrids
- Demonstrated pH-sensitive drug release, with minimal release at physiological pH (7.4) and substantial release in acidic environments (pH 2-5), mimicking the tumor microenvironment
- Achieved nearly complete drug release (99.5%) at pH 4 under NIR exposure
- Confirmed biocompatibility with no adverse effects on mice over 18 days
- Demonstrated significant tumor size reduction (~81.1%) and effective temperature elevation (47.9°C) in tumor tissues under NIR-triggered therapy
- Showed high cell-death rates (~89%) in cancer cells
The technology has been successfully tested in vitro.
This technology represents a major advancement in cancer therapy in minimizing the side effects typically associated with cancer treatments such as chemotherapy and radiation therapy. It offers more precise and effective treatment of solid tumors by ensuring targeted drug delivery and reducing systemic exposure – potentially leading to better therapeutic outcomes, while minimizing damage to healthy tissues.
Superior imaging leads to accuracy of cancer diagnoses and effectiveness of subsequent treatments. Early and precise detection of tumors can lead to better prognoses and treatment outcomes.
This innovation's ability to improve imaging, drug delivery efficiency, and targeted treatment makes it a valuable tool in advancing cancer treatment and diagnosis, while potentially benefiting fields such as nanomedicine and biotechnology.
Geography of IP
Type of IP
202121021279
444111