The invention relates to the creation of hydrogels from poly-amino acids designed from β-aggregation-prone regions of functional amyloidogenic proteins. These designed peptides self-assemble into a three-dimensional nanofibril matrix, providing a robust and biocompatible scaffold for tissue engineering. The hydrogels can be used for 3D tumor models, enabling more accurate drug testing and dosage optimization. Additionally, these hydrogels serve as a depot for the controlled and sustained release of therapeutics and biologic agents, enhancing the efficacy of drug delivery systems. The technology offers a scalable, cost-effective solution with wide-ranging applications in biomedical fields.
Current hydrogel technologies face significant limitations in terms of biocompatibility, biodegradability, and functional versatility. Many hydrogels used for biomedical applications do not adequately mimic the natural extracellular matrix. Conventional two-dimensional cell cultures for testing the effects of anticancer agents are simple and convenient but present significant limitations in reproducing the complexity and pathophysiology of in vivo tumor tissue. The development of three-dimensional (3D) tumor models and effective drug delivery systems is hampered by the inability to create stable, biocompatible hydrogels that can encapsulate and release therapeutic agents in a controlled manner. Existing technologies also struggle with issues such as immunogenicity, complex manufacturing processes, and high costs, which impede their widespread adoption and clinical application.
- Non-Toxic and Low Immunogenicity: The invention produces hydrogels that are inherently non-toxic and elicit low immunogenic responses, making them highly suitable for medical applications.
- Cost-Effective Manufacturing: These amyloid-origin hydrogels can be synthesized in bulk without complex procedures, significantly reducing production costs.
- Simplified Spheroid Formation: Unlike existing methodologies, the hydrogel does not require additional biophysical or biochemical cues to form spheroids.
- Controlled and Sustained Release: The invention ensures a controlled and sustained release of therapeutic agents over time, enhancing the efficacy and duration of drug treatments.
- Thixotropic Nature: The hydrogels exhibit thixotropic properties, making them ideal candidates for long-acting storage depots that do not require complicated drug loading procedures.
- Injectable and Implantable: The hydrogels can be used as injectable drug delivery systems, minimizing surgical risks and enhancing patient compliance.
- Tunable Physiochemical Properties: By modifying amino acids, the physiochemical properties of the hydrogel can be precisely tuned, enabling the engineering of various nanostructures for targeted drug delivery.
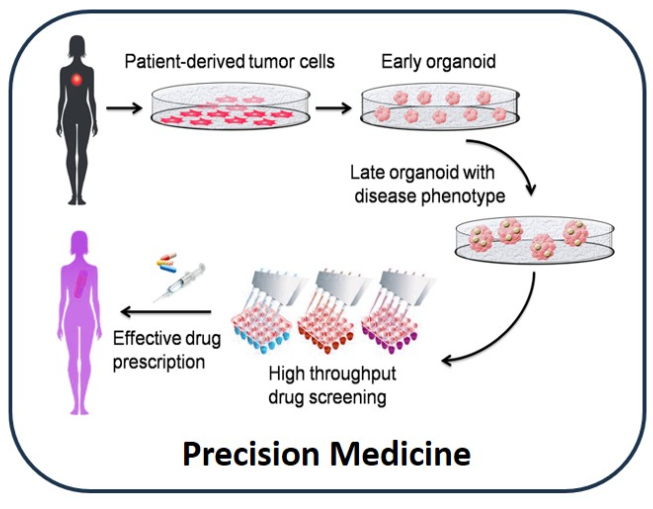
- 3D In Vitro Tumor Models: The hydrogels serve as scaffolds for creating 3D in vitro tumor models, providing a realistic environment for testing anticancer therapeutics and better simulating the native tumor microenvironment.
- Improved Drug Testing Platforms: These hydrogels provide a reliable platform for generating in vitro results that closely mimic in vivo conditions, enhancing the predictability and reliability of anticancer drug testing.
- Minimized Surgical Risks: The injectable nature of the hydrogels reduces the need for surgical delivery of scaffolds, thereby minimizing associated risks and improving patient outcomes.
- Compatibility with Various Cancer Cell Lines: The technology supports the development of 3D tumor models using any cancerous cell line, facilitating comprehensive drug testing from target identification to safety assessment.
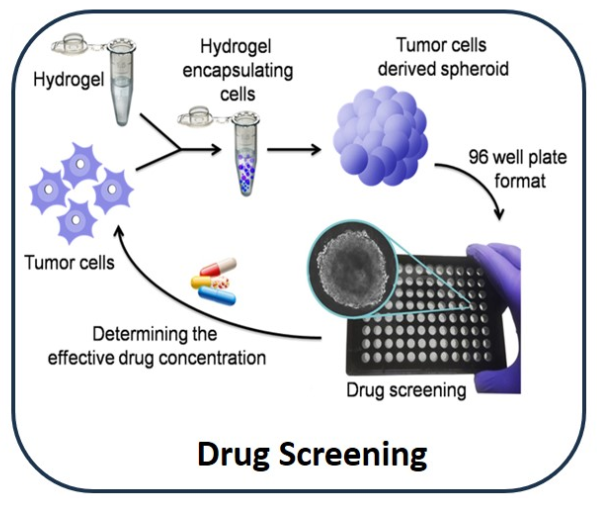
It has shown efficient formation of cancer cell spheroids within 7 days, ranging from 50-300 μm. The technology enables the encapsulation of drugs like doxorubicin and trans-retinoic acid without chemical cross-linking, achieving controlled and sustained release for targeted therapeutic effects.
In a lab environment, successful in vitro tests have demonstrated 80% cancer cell death and neuronal differentiation. Cancer cell spheroids ranging from 50-300 μm were efficiently formed.
5
This technology has the potential to revolutionize biomedical applications by providing more effective and biocompatible materials for tissue engineering and regenerative medicine. It could significantly improve cancer treatment outcomes through better drug testing platforms and more efficient drug delivery systems, ultimately enhancing patient care and treatment efficacy.
- Tissue Engineering and Regeneration: Provides scaffolds for cell adhesion and tissue growth, mimicking the natural extracellular matrix
- Biocompatible Drug Carriers: Reduces adverse immune responses due to their natural peptide composition
- Cancer Research: Facilitates the development of realistic in vitro 3D models for cancer research Implantable
- Medical Devices: Provides biocompatible coatings that reduce rejection and infection risks
- Injectable Hydrogels: Suitable for minimally invasive surgeries and treatments, as they can gel in situ
Geography of IP
Type of IP
201921000523
455242