Today cancer has been identified as one of the deadliest diseases that mankind is combating with. This is certainly curable and there exists a variety of treatment owing to the diversity and intensity of the problem.However, most of these treatments yield fruitful results only when the patients are treated at a rather early stage. This leaves with a major challenge of cancer detection which detection, there are several methods and protocols. Some of these include tumor marker method, cell morphology method and cellimaging. In regard to this problem, we were able to address the fluorescence imaging of cancer cellsusing our expertise in the design and synthesis of glycoconjugates.
The glycoconjugates, when designed accordingly, would not only offer the advantage of water solubility and bio-compatibility, but also cell directing property depending upon the receptor sites present on cancer cells (vs. healthy cells) and their complimentarity with the glycose portion present in the conjugate. The conjugates should be designed appropriately to have a fluorescent moiety or a pre-fluorescent moiety (which will fluoresce when something binds or when something reacts) to have positive results when these interact with cells. The presence of glycose moiety further reduces the toxicity of the fluorescent probe. This means that the design must take care of the integration of all these units in an appropriate manner so that the conjugate can fluoresce inside the cell and can be imaged by the fluorescence microscopy method. An added advantage for the use of such conjugates is that these are amenable to attach even the drug molecule and target its delivery, though we have not carried out the latter function.
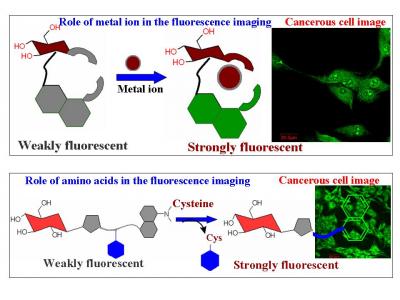
Our group synthesised different glycoconjugates and demonstrated their fluorescence emission behaviour in buffer medium and then extended the studies to cancer cells. Of these, four glcoconjugates were shown to enter the HepG2 cancer cells and were imaged by fluorescence microscopy. One of these conjugates gives green fluorescence emission (~490 nm) in cells, which is quenched in presence of Cu(II) ions. In another case, inside the HepG2 cells, green fluorescence emission (~510 nm) occurred when activated with La(III) ions. In the third case, the cysteine present inside the cells reacted with the probe conjugate and released greenish-yellow fluorescent moiety (~550 nm). The fourth glycoconjugate was designed in such a way that it reacted only with selenocysteine selectively and not with simple cysteine, even though the concentration of cysteine was several order greater than that of Se-cysteine (owing to their nucleophilicity differences). In case of the present conjugate, the HepG2 cells supplemented with the selenium containing compounds exhibited greenish- yellow fluorescence inside the cells. Through this research, we have developed glycoconjugates which enter the cells and provide fluorescence emission under different cellular conditions which was demonstrated with HepG2 cells. This work may find possible applications to diagnose cancerous tissue when the studies are appropriately upgraded to suit tissue conditions.
Prof. Apurba Laha
