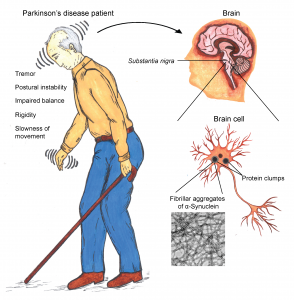
Parkinson’s disease (PD) is a common neurological disorder prevalent mostly in elderly people. This is characterised by progressive loss of brain cells/neurons in specific regions (substantia nigra) of the brain resulting in gradual decline in the sufferer’s motor skills. Mutations in protein, α-synuclein is associated with rare early onset familial forms of PD. The presence of abnormal clumps (aggregates) of the same protein in the neurons surviving in affected regions of PD patients’ brains indicate the key role of α-synuclein in the pathogenesis of PD. Researchers have not been able to pinpoint the specific factors that cause the aberrant clumping of α-synuclein resulting in diseased condition, a fact posing the biggest problem in the field of PD.
Our research group is trying to address this problem from several different directions by working on diverse projects focussing on different aspects of α-synuclein. To begin with, we simulated the disease related protein aggregation process in test tube using human α-synuclein isolated from bacteria.
We found the presence of a new intermediate with unique structural properties and high toxicity towards neurons, which has not been detected before during α-synuclein aggregation process. Further, we found that the familial PD associated mutations cause local changes in the structure of α-synuclein both in its native as well as aggregated forms, which could be correlated to differential disease pathology in familial mutation harbouring individuals. Hence, our studies are important steps towards gaining deeper insights into the toxic mechanisms underlying PD. We are also screening small molecules which modulate α-synuclein aggregation and thus, may be used as potential candidates for drug development process against PD.
Prof. Samir K Maji
