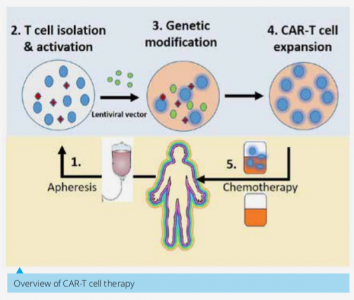
Chimeric Antigen Receptor-T cell therapy (CAR-T cell therapy) has demonstrated remarkable success in long-term remission of relapsed or refractory B-cell precursor acute lymphoblastic leukemia (B-ALL). Very recently, FDA approved the anti-CD19-CAR-T cells developed by Novartis for the treatment of B-ALL. However, this technology has not yet been designed and developed in India. Considering socioeconomic conditions of patients in our country, recently developed CAR-T-cell therapy will be unaffordable to majority of our patients. To harness this technology and bringing it to the clinic in India at affordable-cost, there is a clear need of developing indigenous CAR-T cell technology platform.Recently, we developed the CAR-T cell platform and testing their efficacy at laboratory scale. Next logical step is to translate the CAR-T cell technology at clinical stage, which requires infrastructure building and industry-academia partnership to successfully brings this technology to the patients benefit.
Prof. Rahul Purwar
