High mortality in cancer is attributed to cancer metastasis–the multi-stage process which includes invasion of tumor cells through the basement membrane, entry into blood vasculature, entry into secondary organ and its colonisation. Understanding mechanisms of cancer invasion represents one of the major research directions of our group. For this we combine traditional cell and molecular biology with biophysics and computational biology. We are currently working on the following aspects:
Modes of cancer invasion
Cancer invasion is mediated to a great extent by members of the matrix metalloproteinases (MMPs) which degrade the extracellular matrix thereby creating paths for cell invasion. Interestingly, inhibition of MMPs does not stop cell invasion. Instead, cells switch from a protease dependent mesenchymal mode of invasion to a protease independent mode amoeboidal of invasion, referred to as mesenchymal to amoeboidal transition (MAT). We are probing the biophysical alterations in cell/nuclear properties that accompany MAT.
Cell mechanics
Cancer invasion requires cancer cells to squeeze through pores in the extracellular matrix as well as through the endothelium. This requires cells to alter their cytoskeletal architecture leading to alterations in cell mechanical properties as well as generation of increased cell matrix tractions that mediate nuclear deformation. We quantify mechanical properties of single cells, correlate their mechanical properties with invasiveness, and probe the role of specific cytoskeletal proteins in modulating cell invasiveness.
Role of cytoskeletal proteins in mediating therapeutic resistance
One of the reasons late stage cancer in difficult to treat is due to development of therapeutic resistance. We are probing the role of cytoskeletal proteins in mediating drug resistance and radiation resistance. Recently, we have identified a-actinin-4 as an important protein that contributes to radiation resistance in breast cancer.
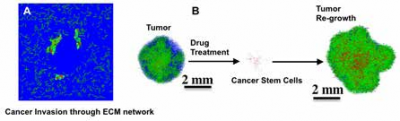
Computational modeling of cancer invasion and tumor heterogeneity
Given that cancer invasion involves multiple processes occurring across different length scales and time scales, it is often difficult to understand the phenomenon using simple experimental setups. For this purpose, we develop computational models that incorporate multiple aspects of cell behaviour and properties of the extracellular matrix for understanding the roles of cell intrinsic and extrinsic properties in regulating cancer invasion (Fig. 1A). We are also developing models of tumor heterogeneity in order to understand how physical factors dictate the emergence of phenotypic heterogeneity and its implications (Fig. 1B).
Prof. Shamik Sen
