The human body is bordered by several cellular barriers that define the frontier between the internal milieu and the external non-sterile environment. In addition to their physiological roles, these host barriers provide both physical and immune defense against microbial infection. Yet, many pathogens have evolved elaborate strategies to target these lines of defense, resulting in disruption of barrier integrity and dissemination into deeper tissues. Employing blood brain barrier (BBB) and fetematernal barrier as model barriers and Group B Streptococcus and Streptococcus pneumoniae as model pathogens, we strive to decipher the novel mechanisms adopted by these pathogens to breach host barriers.
Amongst the few microorganisms that can efficiently breach the BBB, Streptococcus pneumoniae (SPN) is unique in a sense that most humans are asymptomatic carriers of this bacterium in the respiratory tract. But due to reasons still unknown, this bacterium switches to a pathogenic state and can
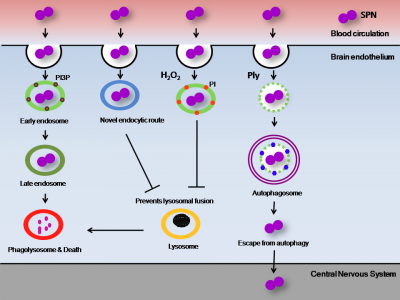
cause a wide range of diseases including meningitis, a serious infection of the central nervous system. It has been demonstrated that SPN invades the 8 BBB and is capable of transcellular passage through the brain endothelium, but the exact mechanisms of intracellular trafficking and survival are poorly understood. Typically, endocytosed bacteria would be trafficked through endosomes until eventual destruction in the lysosome. Internalised SPN therefore must avoid delivery to degradative lysosomal compartment for efficient transit across BBB. Our findings reveal that by expressing different virulence factors, such as pore forming toxin pneumolysin or hydrogen peroxide, SPN alters host cellular pathways such as autophagy to promote its survival and efficient transit across BBB. We also discovered that SPN adopts a novel endocytic route to enter BBB which no other pathogen is known to employ, preventing its eventual killing in lysosome (Fig. 1).
Future work in this direction include: (1) Development of novel therapeutic approaches that prevent bacterial resistance to cellular killing mechanisms and (2) Serotype independent vaccine preparation for pneumococcus to reduce its disease burden.
The other major interest of our research group is in Group B Streptococcus (CBS), an opportunistic pathogen that resides in the genito-urinary tract of nearly 20-30% women. This carriage is associated with increased risks of complications during pregnancy such as chorioamnionitis (inflammation of the membranes that forms the barrier between mother and fetus) and premature rupture of membranes (PROM) subsequently leading to preterm birth. Although ascending infection of CBS from vagina into uterus accounts for many instances, in a large proportion of cases, the bacterium is nowhere present near these membranes. We discovered that CBS produces small membrane bound vesicles (MVs) loaded with proteases and other virulence factors while colonising female genital tract. These vesicles can travel up the birth canal and reach the uterus where they can degrade collagen, a protein that provides strength to feto-maternal membranes to bear the weight of growing fetus. They also cause extensive inflammation and cell death of feto-maternal membranes. The culminating effect of such adverse events leads to rupture or fetal membrane, premature delivery and / or intrauterine fetal demise in majority of cases (Figure 2). This provides a plausible explanation for how CBS residing in vagina/ rectum can coordinate events at the
feto-maternal barrier leading to preterm birth. Ongoing projects in this direction include: (1) Investigating the rate of GBS colonisation amongst Indian women and its correlation with preterm birth and other pregnancy complications, (2) Studying the effect of CBS MVs on fetal physiology, specifically, neuro degenerative disorders and cognitive deficits and (3) Exploring the possibility of membrane vesicles as potential vaccine candidates for GBS infection.
Prof. Anirban Banerjee
