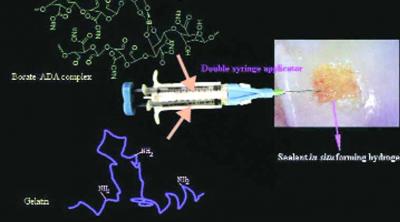
Osteoarthritis is a progressive musculoskeletal degenerative disorder with clinical symptoms of joint pain characterised by the gradual loss of articular cartilage and inflammation of the joint. According to World Health Organisation statistics, about 9.6% of men and 18% of women aged over 60 years have symptomatic osteoarthritis and 80% of those with osteoarthritis will have mobility problems restricting them from performing major daily activities of life. In addition, injuries also lead to damage to the articular cartilage. At present there are no clinically approved minimally invasive ways of cartilage replacement and most cases require joint replacements.The solution developed is an injectable, in situ gelling hydrogel which is biocompatible and adhesive binding with host tissue. This functions as a cushion to replace the articular cartilage. The material mimics then extracellular matrix of the cartilage and is specially designed to have a lowviscosity on administration, which triggers to form a cross linked gel locally at the site of administration. The technology consists of biodegradable FDA approved materials and promotes regeneration of the cartilage cells (chondrocytes) allowing the maintenance of the functional hyaline cartilage phenotype as desired. Various prototypes of the technology are ready for them different stages of arthritis. The technology has been validated in vitro and in cellular models. The next steps are translation in small animal models, toxicology evaluation and scale up manufacturing. This technology has the potential to address the underlying cause of the symptoms of osteoarthritis.
(Late) Prof. Rinti Banerjee
